Essential reading for intensivists: things you didn't even dream were going on in your ventilated patients' lungs. With credit to @robertpdickson who does some very clever translational work on lungs
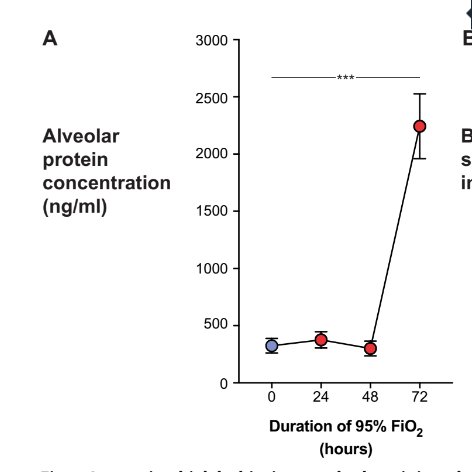
Firstly, we are aware of the inherent badness of oxygen. Here is protein content in alevoli after 48 hours of FiO2 95% (i.e. oedema), delightful.
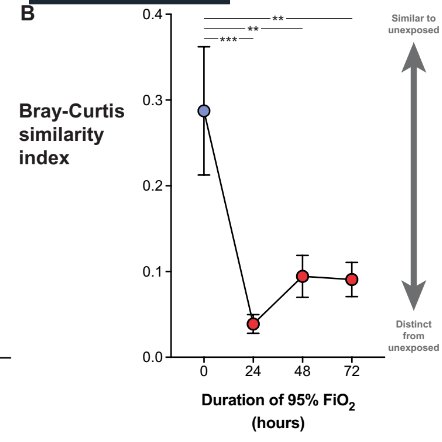
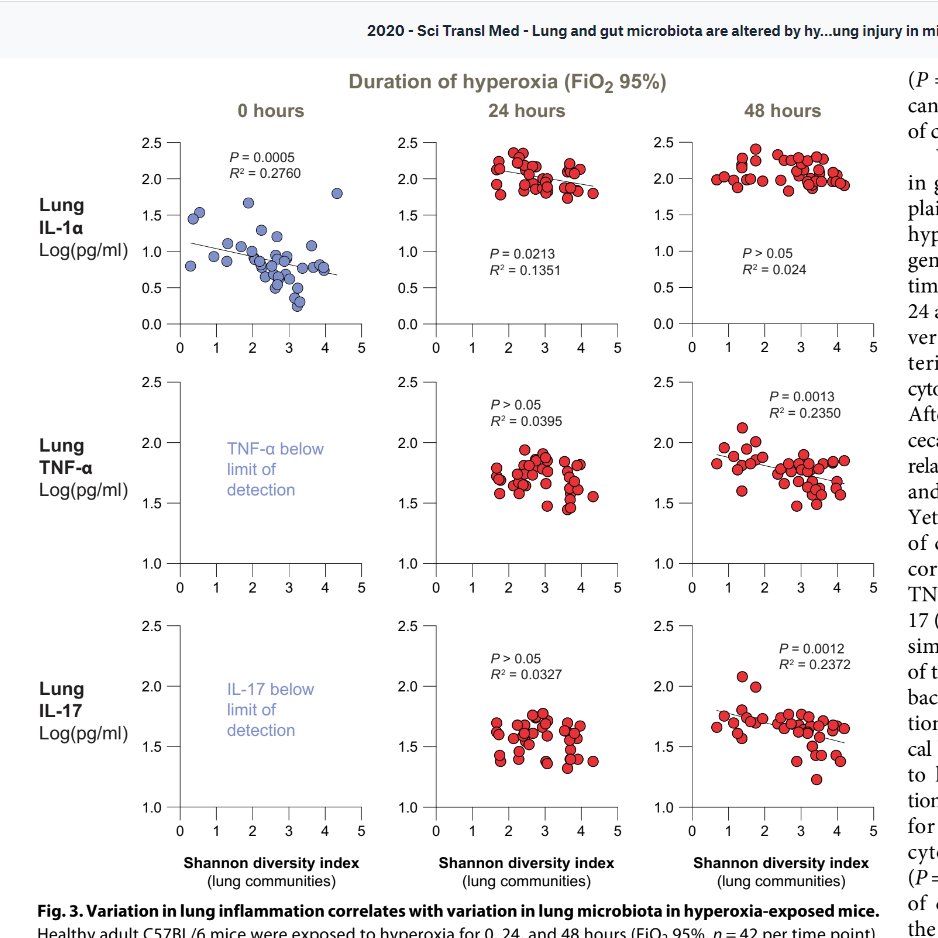
Secondly, all those VAP's we get cross with (can someone silver impregnante the ET tube or something) that we think is just orally communicating flora/immunosuppression? Mais non. High FiO2 all but destroys weakling bacteria, leaving the redox-armoured king, staph aureus, to rule
Unmitigated staph surges and produces a truckload of PAMPs (pathogen associated molecular patterns). Being redoxically armoured, it is fairly happy to thwart oxidative burst from neutrophils attempting to kill them too.
Indeed the lung flora changes prior to the alveolar protein content - like both a canary in the coalmine moment, and then contributing to pathology.
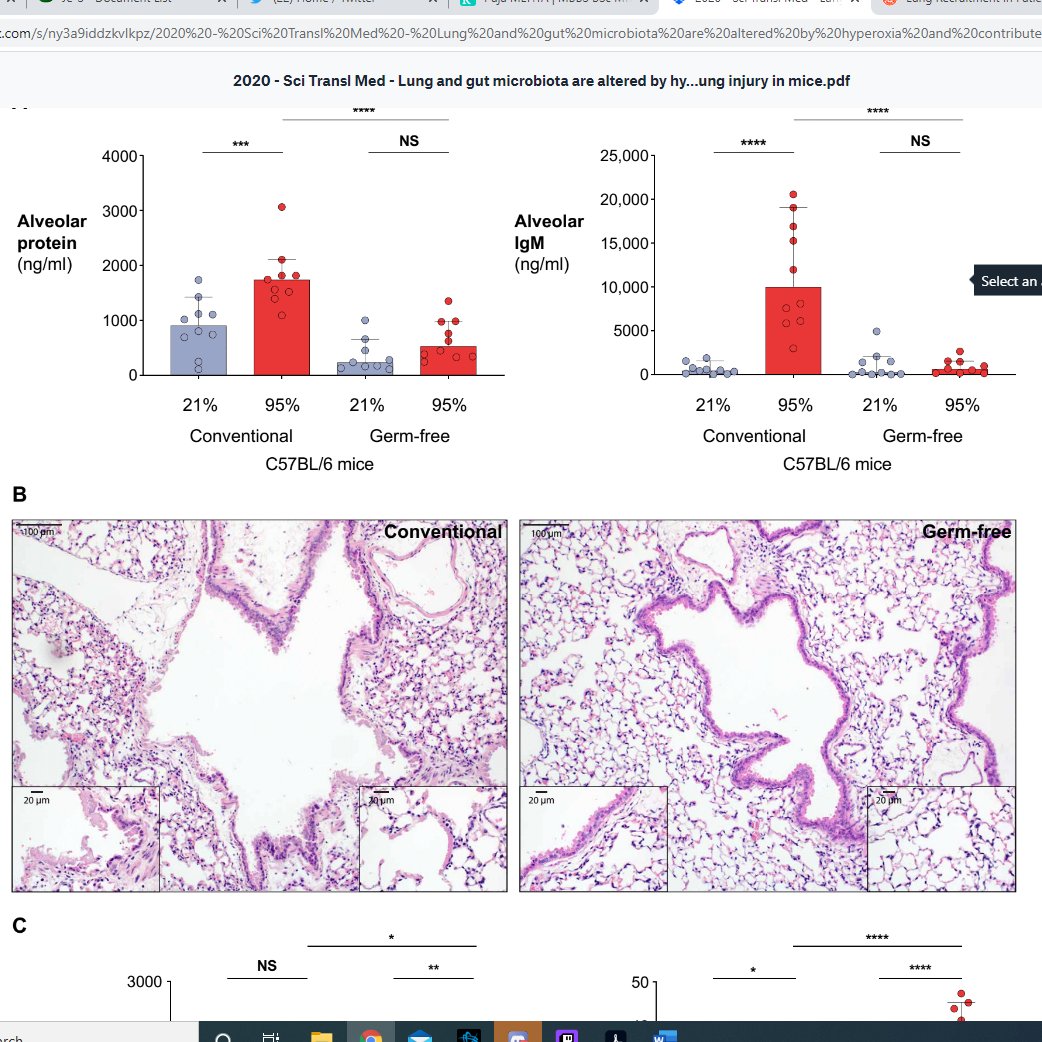
So, we have been breeding oxidation resistant pneumonia, brilliant. Does that mean people above a certain FiO2 should get prophylactic antibiotics? I mean, germ free mice don't get the same severity lung injury...
Graphs below: show less alveolar protein/immunoglobulin after 48-72 hours of FiO2 95%, and more nicely retained alveolar histology in germ free mice
On the other hand, as if that wasn't confusing enough, there is also a problem with gut microbiome and lung injury. How upsetting.
The gut microbiome changes later than the lung.
The gut microbiome changes later than the lung.
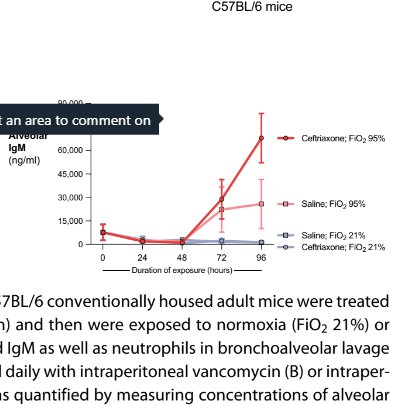
If you do give systemic antibiotics (this study used ceftriaxone and vancomycin all of which we use in ITU in UK) you make things much worse :O
BUT WHY YOU JUST TOLD ME STAPH WAS TAKING OVER AND NEEDED KILLING. Well, when you give these antibiotics you especially get rid of clostridia species in lung and gut. In gut, clostridia provides butyrate for colonocytes. When they don't get butyrate
they start to respire anaerobically, leaving gut lumen oxygen higher, meaning enterobacteriae go whoosh, and take over gut. In lung, staph goes whoosh. And no doubt it's the ceftriaxone resistant version of staph that does.
As a side note, the mechanisms that staph use to resist oxidative damage are the ones we use - superoxide dismutase, thioredoxins, flavins, DNA protection. But we only have this sufficiently available during the DAY and we don't have good anti-ox at NIGHT
which is why bad idea to have big brain bleed full of oxygenated Hb at NIGHT because the heme and the oxygen mess you up, especially badly.
Original paper here https://stm.sciencemag.org/content/12/556/eaau9959.abstract
As usual, understand less than when I started, about good lung care in ICU.
starting point for circadian difference sub arach https://www.ahajournals.org/doi/full/10.1161/strokeaha.116.016165
starting point for circadian difference in bleomycin toxicity https://www.pnas.org/content/117/2/1139

 Read on Twitter
Read on Twitter