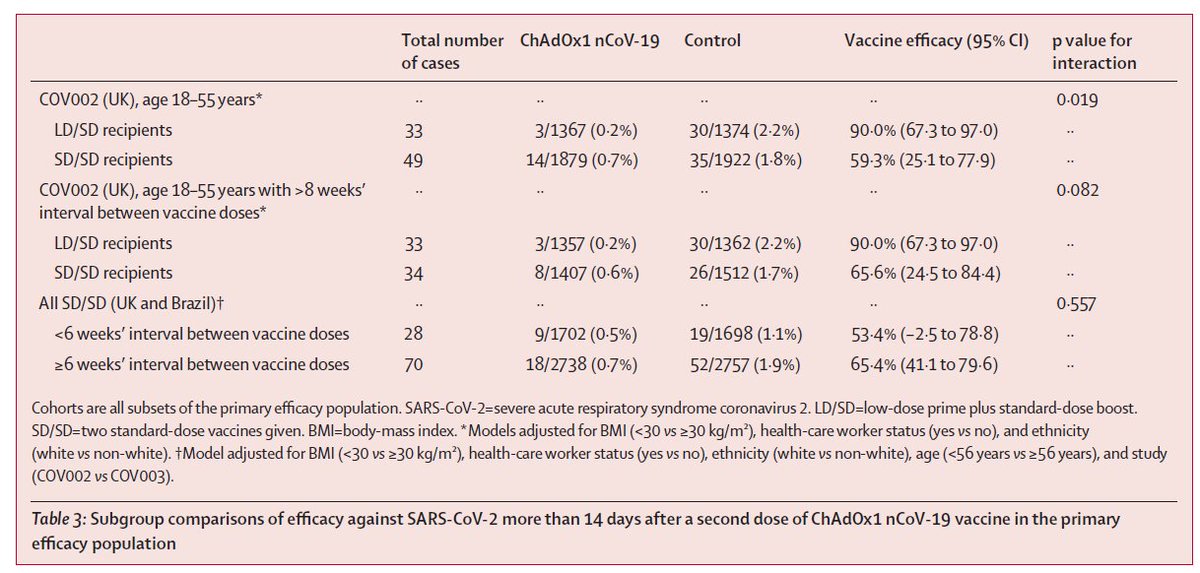
Out in @TheLancet, results from the Oxford/AZN trials, including more detail on the low dose results. Notably, the low dose recipients "received their second dose after a substantial gap." Only 0.8% received a second dose within 8 weeks of the first. 1/5
https://www.thelancet.com/lancet/article/S0140-6736(20)32661-1
https://www.thelancet.com/lancet/article/S0140-6736(20)32661-1
Recall that the low dose results were only from adults 18-55, only during a short time window, and only in the UK. Per reviewer request, they restricted the standard dose analysis to a similar group. We still see separation (middle rows), but with more uncertainty. 2/5
Overall, the 62% result for the standard dose regimen appears robust, and meets pre-specified criteria (>50%). But I am still not sure what to make of the low dose result. Is it the longer gap between doses? The low dose? Both? And there remains no data for older adults. 3/5
Per the commentary, we will need more prospectively collected data on the low dose group, both to confirm a true biological effect, and to generate safety/efficacy data in older adults. Remember, these vaccines have big-time policy implications. 4/5
https://www.thelancet.com/lancet/article/s0140-6736(20)32623-4
https://www.thelancet.com/lancet/article/s0140-6736(20)32623-4
A final comment.... the paper is complicated. I fully recognize the complexities of doing science in a pandemic, but there is something to be said for standardization of trial protocols in advance, transparency, and simple study methods. Makes for a cleaner result. 5/5

 Read on Twitter
Read on Twitter