COVID19 causes FLORID platelet activation, FAR beyond what ARDS typically causes. Platelet hyperreactivity in COVID19 is quantifiably higher than typical ARDS and exorbitantly higher than healthy individuals or admitted inpatients.
This platelet hyperreactivity and platelet derived mediators including serotonin define COVID19's pathology, in my humble opinion.
ssRNA activation of platelets to act as IMMUNE cells (independent of thrombotic action) is well described and occurs very commonly.
ssRNA activation of platelets to act as IMMUNE cells (independent of thrombotic action) is well described and occurs very commonly.
This ssRNA, SARS-CoV-2, happens to access body's vasculature via alveoli given its ACE2 and other receptor capacity to injure alveolar cells, and pericytes of endothelium.
Other ssRNA (Dengue) happen to reach vasculature and activate platelets via mainly other routes.
Other ssRNA (Dengue) happen to reach vasculature and activate platelets via mainly other routes.
50% of total body platelet are produced within lung microvasculature.
This vasculature is filled with resident megakaryocytes and platelets.
This vasculature is filled with resident megakaryocytes and platelets.
Lung microvasculature injured and infected by SARS-CoV-2 now not only harbors activated platelets via virus's direct action (via TLR7, etc) but also harbors, importantly, a destabilized endothelium, via viral action of ACE2 on endothelial pericytes leading to their apoptosis.
Pericytes are the equivalent of smooth muscle cells of capillaries, and injured pericytes lead to a destabilized pulmonary endothelium, and endotheliopathy that occurs far more intensely in COVID19 ARDS than influenza ARDS, per NEJM by Ackerman.
This endotheliopathy directly leads to an exponential further activation of already virally-activated platelets within pulmonary microvasculature in COVID19 ARDS.
So a good question is, why are we not putting patients on antiplatelets to get to the core actor of the carnage caused in COVID19 ARDS, the end result of which (actual thrombi) we are treating with anticoagulation with some success.
This is the reason that full dose anticoagulation and tPA mainly “clean” the crumbs (thrombi) in pulmonary microvasculature but don’t address the source of the problem, the hyperreactive platelets and megakaryocytes due to the abovementioned viral effect.
Expectedly, the platelet derived mediators continue to be released floridly and these mediators, not the actual thrombi necessarily, form the basis of not only further thrombi, but also the vast majority of injury with potent vasoconstriction of distal pulmonary vessels.
Expectedly, the precapillary arterioles release their natural anti-platelet anti-constrictive mediator, PGI2, to counteract the carnage they see distally in these capillaries.
This released PGI2 and other endogenous vasodilators (iNO) end up causing proximal reactive vasodilation in the pulmonary pre-capillary vasculature, seen repeatedly on CT in COVID19 ARDS.
Platelet-released mediators include highly bronchoactive and vasoactive mediators such as 5HT, among others, which we will get to in a minute.
Platelet-released mediators also include angiogenesis factors (PDGF, VEGF) that lead to further rapid development of angiogenesis in these injured capillaries, as a proper reaction of the body to divert the flow-obstructed pulmonary circulation to its eventual destination.
These eventually form the basis of intrapulmonary Right to Left shunts, shown in studies by Poor et al, and this is the basis of the "happy" hypoxemia that responds to prone position rapidly, a response similar to what's seen in hepatopulmonary syndrome.
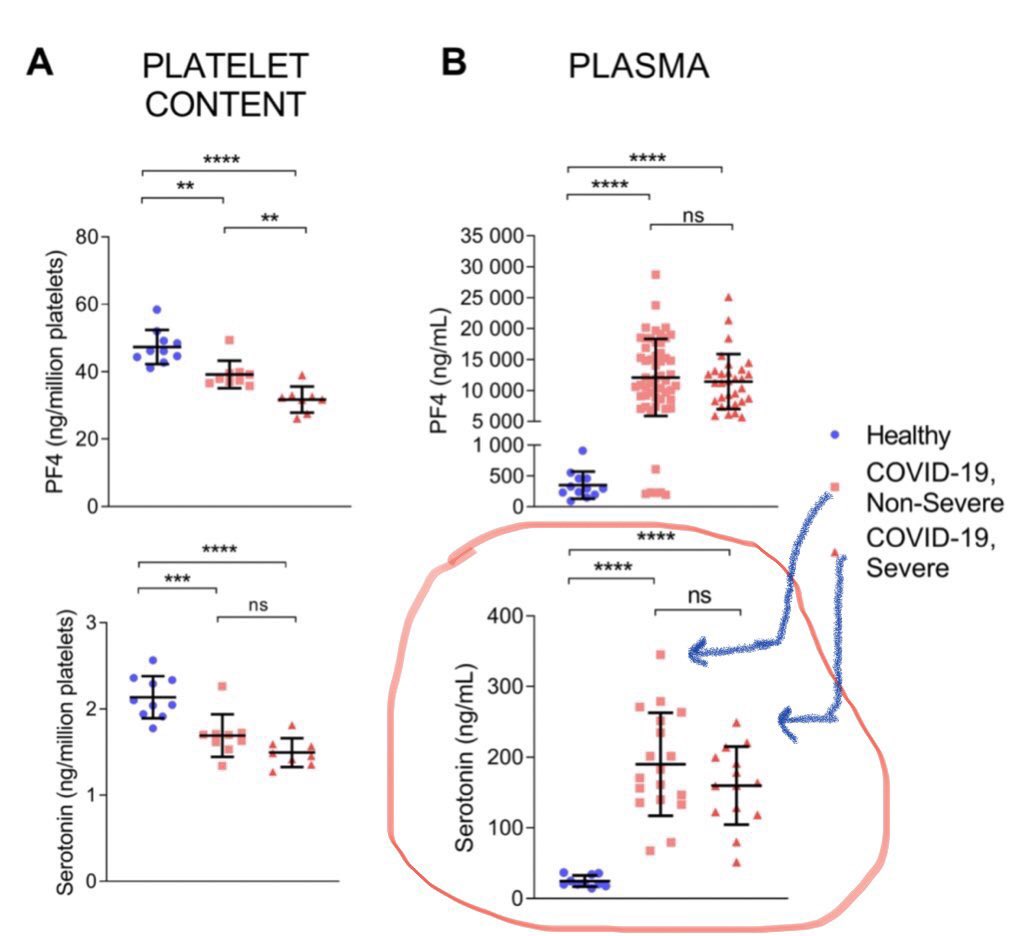
Platelets also contain 95% of the body's total content of serotonin (5HT).
Plasma is maintained void of free circulation 5HT in a tightly regulated manner to prevent the toxic effects of free 5HT that we occasionally see in Serotonin Syndrome.
Plasma is maintained void of free circulation 5HT in a tightly regulated manner to prevent the toxic effects of free 5HT that we occasionally see in Serotonin Syndrome.
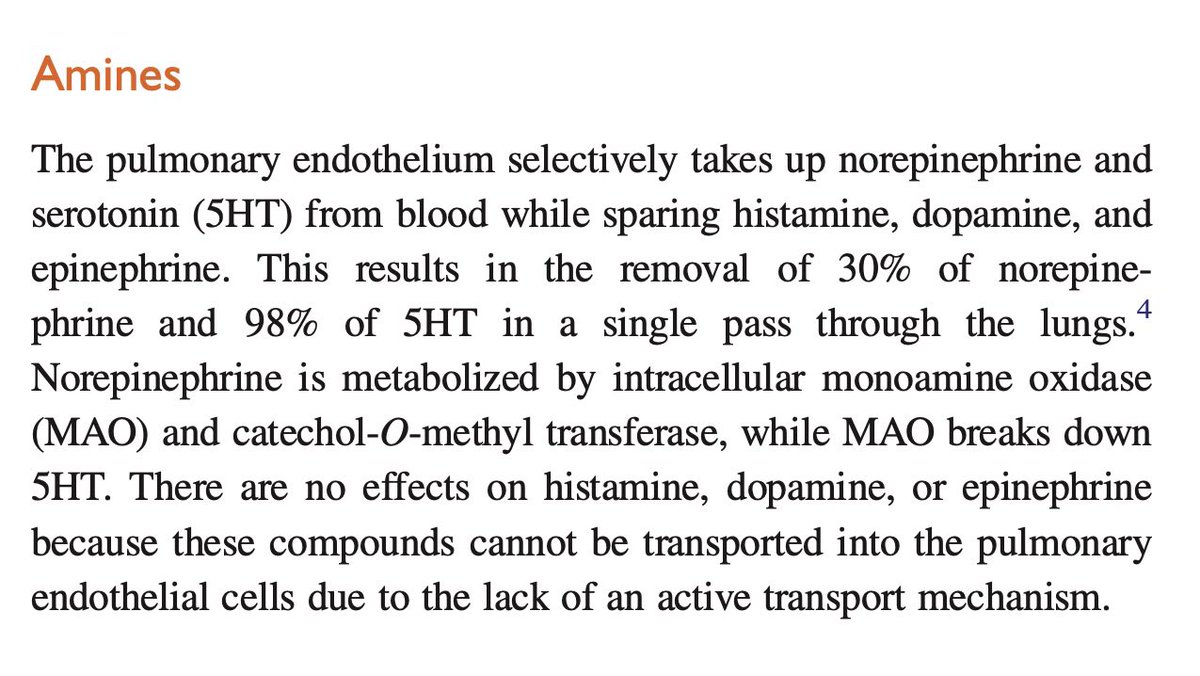
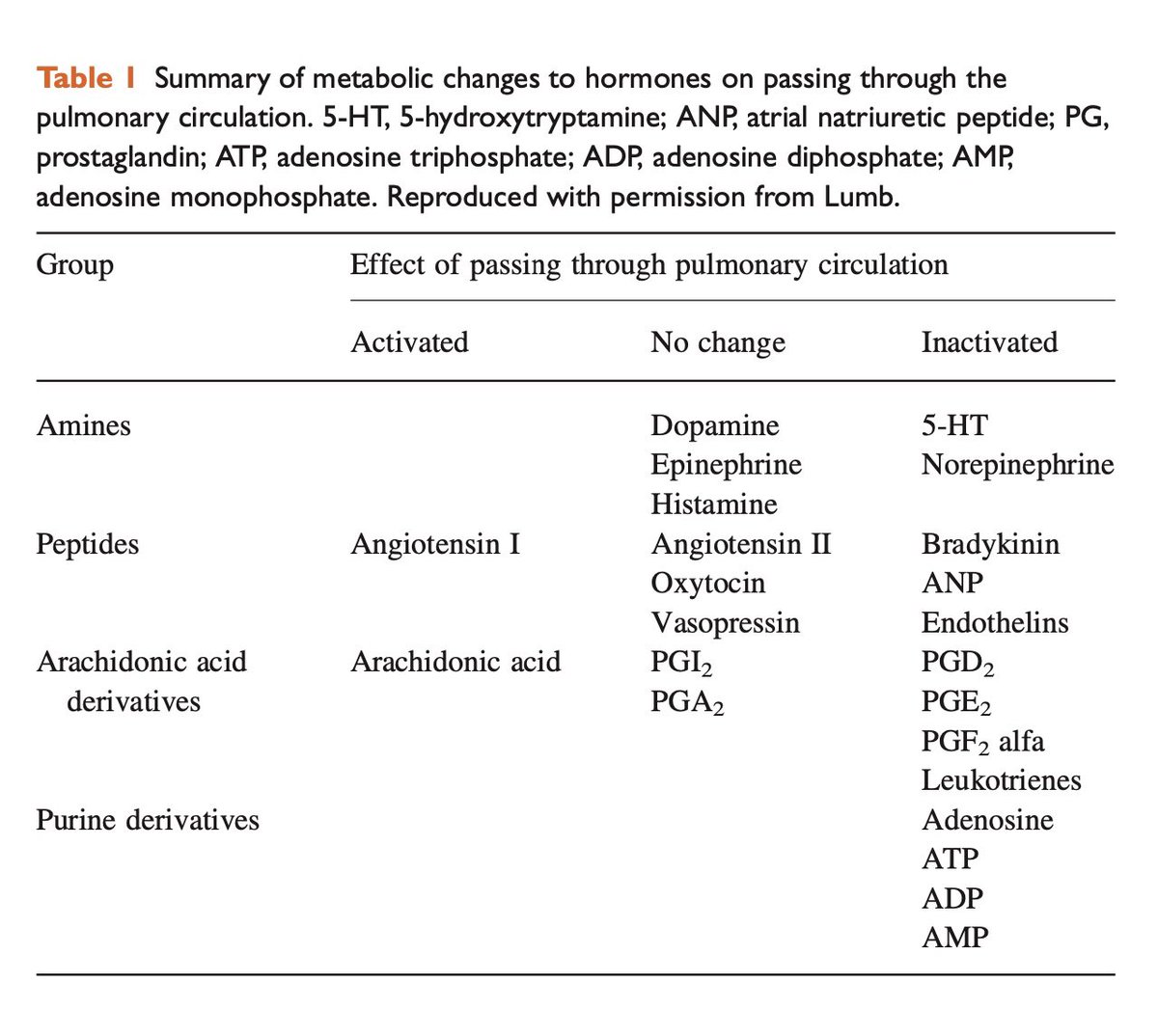
Lung microvasculature are responsible to clear the free plasma 5HT, when released due to any clotting action or other processes such as overdose on serotonergic medications, etc.
At least 80% of this clearance of 5HT is dependent on healthy lung endothelium.
At least 80% of this clearance of 5HT is dependent on healthy lung endothelium.
In COVID19 ARDS, the activated and hyperreactive platelets within pulmonary microvasculature release 5HT, as they should.
However, the co-existing pulmonary endothelial injury leads to a deficiency in 5HT reuptake and soon a vicious feed-forward cycle of 5HT buildup will form.
However, the co-existing pulmonary endothelial injury leads to a deficiency in 5HT reuptake and soon a vicious feed-forward cycle of 5HT buildup will form.
This vicious cycle will form more rapidly if a higher proportion of lung alveoli and hence lung endothelium is exposed to the virus, as in high inoculum.
Lung endothelial injury is also a common side effect of ventilator induced lung injury.
Let me now explain why a high dead-space (high platelet activation and thrombi) injury as in COVID19 ARDS leads to a higher risk of ventilator induced lung vascular injury.
Let me now explain why a high dead-space (high platelet activation and thrombi) injury as in COVID19 ARDS leads to a higher risk of ventilator induced lung vascular injury.
The dead space created due to this platelet activation in COVID19 ARDS leads to compensatory alveolar duct constriction.
Imagine the lung doing exactly what it is supposed to do to reduce gas exchange issues.
Imagine the lung doing exactly what it is supposed to do to reduce gas exchange issues.
If an alveolus has poor perfusion (due to platelet-rich clots, and platelet-derived vasoconstriction from 5HT and other mediators), then this alveolus does not deserve to get the same amount of ventilation as the rest of the lung.
This alveolus, in a process that is healthy and compensatory, undergoes a reaction where the lung tells the alveolar duct (the air channel into alveolus) to shut close. This occurs via platelet-derived 5HT.
Essentially, the alveolus closes and stays actively shut.
Essentially, the alveolus closes and stays actively shut.
This diverts the ventilation toward healthy alveoli without a vascular injury. This reduces what's called high V/Q mismatch.
This is healthy and 100% occurs in any alveolus with dead-space.
This is healthy and 100% occurs in any alveolus with dead-space.
However, these alveoli with dead-space-induced alveolar duct constriction have very little capacity to be recruited (inflated) given the active constriction.
On the other hand, the normal alveoli without platelet activation (without viral injury) are normally distended.
On the other hand, the normal alveoli without platelet activation (without viral injury) are normally distended.
These healthy alveoli without any dead-space possibly have an increased venteilation routed toward them naturally, as explained above, to reduce high V/Q mismatch.
When you're not intubated, the body tries to handle this with a nuanced approach that probably only body can do.
When you're not intubated, the body tries to handle this with a nuanced approach that probably only body can do.
In the context of mechanical ventilation though, higher driving pressure and higher power will run into few issues.
First, the alveoli with dead-space are constricted and do NOT allow inflation or expansion easily.
First, the alveoli with dead-space are constricted and do NOT allow inflation or expansion easily.
These dead-space-afflicted alveoli will be HARD to recruit. This is not the issue though.
The issue revolves around the healthy alveoli.
The issue revolves around the healthy alveoli.
These healthy normal alveoli, that were distended normally (or slightly higher due to compensation explained above) prior to mechanical ventilation, do not have a whole lot of room to expand further.
These previously healthy alveoli without much room to expand are at a very HIGH risk of ventilator-induced lung injury, due to the risk of hyperinflation or too much air beyond their capacity going into them.
Now it would be something to just have ALVEOLAR injury due to hyperinflation, but the main issue is that the VESSELS within these previously normal alveoli are also highly injured due to hyperinflation.
These previously normal vessels harbored the only healthy endothelium to process and regulate the released 5HT from the remainder of the lung that was virally injured and having an issue with hyperreactive and activated platelets.
Now with these previously healthy alveolar capillaries undergoing injury due to VILI, there is not a whole lot of capacity left to process platelet-derived 5HT.
This leads to a feed-forward vicious cycle of 5HT excess in plasma, in pulmonary circulation.
This leads to a feed-forward vicious cycle of 5HT excess in plasma, in pulmonary circulation.
Eventually this excess 5HT reaches the systemic circulation via the previously mentioned R to L intrapulmonary vascular shunts that develop in the areas with high degree of platelet activation and viral injury.
This is the "Death-rattle" of mechanical ventilation that we initially saw early in the pandemic, with rapid decline and death occurring soon after initiation of mechanical ventilation.
It is basically a very rapid increase in the feed-forward 5HT levels in the body.
It is basically a very rapid increase in the feed-forward 5HT levels in the body.
Excess systemic plasma 5HT leads to hyperpnea or rapid breathing without an actual gas issue to cause it (without hypercapnia).
Similarly, excess 5HT causes renal artery vasoconstriction, the dreaded "renal failure" that occurred commonly after initiation of mech ventilation.
Similarly, excess 5HT causes renal artery vasoconstriction, the dreaded "renal failure" that occurred commonly after initiation of mech ventilation.
This is the reason that most biopsies of kidneys show no serious degree of ATN or even thrombi, and are puzzling to decipher what caused the renal failure, given no major hypotension even occurred in these patients.
The issue is that the renal artery is vasoconstrictor potently due to excess 5HT and this won't be evident necessarily in any biopsy or autopsy, but has been shown actually in a study published in the journal of SHOCK recently.
The other major side effect of excess circulation 5HT is vasoconstriction in another important capillary bed, the brain and central nervous system.
5HT has been repeatedly demonstrated to cause CNS microvascular injury, with blood-brain barrier breakdown, and vasoconstriction.
5HT has been repeatedly demonstrated to cause CNS microvascular injury, with blood-brain barrier breakdown, and vasoconstriction.
This forms the basis of brainstem injury commonly seen in patients with severe COVID19. This essentially is the insult that leads to a very common (64% in one series) pathognomonic symptoms of serotonin syndrome such as hyperreflexia and myoclonus in patients with COVID19.
This also is the reason there are many severe COVID19 patients who are labeled as "hard to sedate" despite very high doses of Versed and Fentanyl and trials of other sedatives.
If serotonin excess is a culprit, in fact, Fentanyl is injurious and leads to further serotonin toxicity. This is a known fact.
So in essence, we are treating these patients with Fentanyl in the hope of better sedating them, but we are actually worsening the issue.
So in essence, we are treating these patients with Fentanyl in the hope of better sedating them, but we are actually worsening the issue.
This could be treated with Cyproheptadine orally and benzodiazepines and anti-histamines such as Pepcid have been shown to effectively treat serotonin toxicity as it pertains to the central nervous system effects.
But more importantly, it's best to avoid this serotonin toxicity by avoiding higher pressure and high power ventilation, to delay intubation as long as safe, and to perhaps avoid Fentanyl and other possible medications with serotonergic activities.
Beyond CNS and renal (two very important organs) effects of excess 5HT, many other organs are affected.
5HT regulates systemic glycemia (and actually regulates CNS/choroid insulin levels, which are separate and not related to systemic insulin levels).
5HT regulates systemic glycemia (and actually regulates CNS/choroid insulin levels, which are separate and not related to systemic insulin levels).
Brittle and fluctuating low and high glucose levels are a common sight in COVID19 and absolutely has ZERO explanation otherwise, something we've rarely seen in other causes of ARDS or even other diseases.
This is due to 5HT excess.
This is due to 5HT excess.
Additionally, 5HT-induced mesenteric non-occlusive ischemia is also well described, in addition to bouts of severe diarrhea that are reportedly commonly in not only severe, but moderate cases of COVID19.
Excess plasma 5HT also leads to vasoplegia systemically, essentially what is sometimes labeled as "shock".
Some of the effects of 5HT are potentiated (worsened) by administration of norepinephrine, but this is not entirely clear-cut and not a ton of evidence exists on this.
Some of the effects of 5HT are potentiated (worsened) by administration of norepinephrine, but this is not entirely clear-cut and not a ton of evidence exists on this.
So essentially, to avoid the above cascade, it's worthy to consider the main culprit, the platelet hyper-reactivity.
Platelet hyper-reactivity occurs in ARDS. It is a known fact.
Platelet hyper-reactivity occurs in ARDS. It is a known fact.
But it occurs with far higher intensity in H1N1 ARDS compared to bacterial pneumonia and other causes of ARDS, as shown in a study by Rondina from Utah.
We are, however, likely witnessing the most potently platelet-activating viral-mediated ARDS in COVID19.
We are, however, likely witnessing the most potently platelet-activating viral-mediated ARDS in COVID19.
To recap, ssRNA are known to activate platelets, as platelets are essentially the immune cells that initiate the immune response in these viral infections.
SARS-CoV-2 happens to access a boatload (=50% of total body content) of platelets that naturally reside in lungs.
SARS-CoV-2 happens to access a boatload (=50% of total body content) of platelets that naturally reside in lungs.
It also happens to, via its ACE2 receptor affinity, to destabilize the pulmonary endothelium, the very very organ that regulates platelet maturation and release from pulmonary circulation to the rest of the body.
It's a double whammy, filled with dead-space pathology.
It's a double whammy, filled with dead-space pathology.
And characterized in its most severe form by platelet-derived mediators. Among all these mediators, one happens to also rely on the same injured pulmonary endothelium to be "cleaned" out of the body. And that is 5HT.
A molecule widely active throughout the lung and the body.
A molecule widely active throughout the lung and the body.
So essentially this is a single organ failure, lung (and its vasculature), with not only gas exchange issues, but also issues stemming from its defined role to regulate platelet maturation and 5HT uptake (to load onto maturing platelets) throughout the body.
... Leading to multisystem organ failure via the wide ranging actions of serotonin excess.
All of above is well researched and references are available.
Perhaps if there was one paper to read on this, I’d say it’s this old study (MGH) in human ARDS:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1250439/pdf/annsurg00114-0060.pdf
All of above is well researched and references are available.
Perhaps if there was one paper to read on this, I’d say it’s this old study (MGH) in human ARDS:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1250439/pdf/annsurg00114-0060.pdf

 Read on Twitter
Read on Twitter