My podcast w/ Paul Offit, one of the world's leading lights on vaccines, regarding #SARSCoV2 vaccine development
https://www.medscape.com/viewarticle/936937 @Medscape
It's rich and I'll summarize key points here
1. "It's an unprecedented moment...as long as the Phase 3 trials don't get truncated"
https://www.medscape.com/viewarticle/936937 @Medscape
It's rich and I'll summarize key points here

1. "It's an unprecedented moment...as long as the Phase 3 trials don't get truncated"
2. Things we're learning about the virus
--not affected by seasons
--can cause a vasculitis
--unusual multi-system inflammatory syndrome in children
--strong predilection for nursing homes
--not affected by seasons
--can cause a vasculitis
--unusual multi-system inflammatory syndrome in children
--strong predilection for nursing homes
3. What about #LongCovid?
4. Can any Phase 3 vaccine trial have a readout by the end of October?
" I don't see how it's possible"
Clinical endpoints are moderate or severed covid disease
" I don't see how it's possible"
Clinical endpoints are moderate or severed covid disease
5. The vaccine trials do not enroll people w/ known prior covid infections. What is the rate of people who are seropositive (determined after enrollment) not having a known infection?
1%
(that's remarkably different cf estimated seropositivity in the US ~15%)
1%
(that's remarkably different cf estimated seropositivity in the US ~15%)
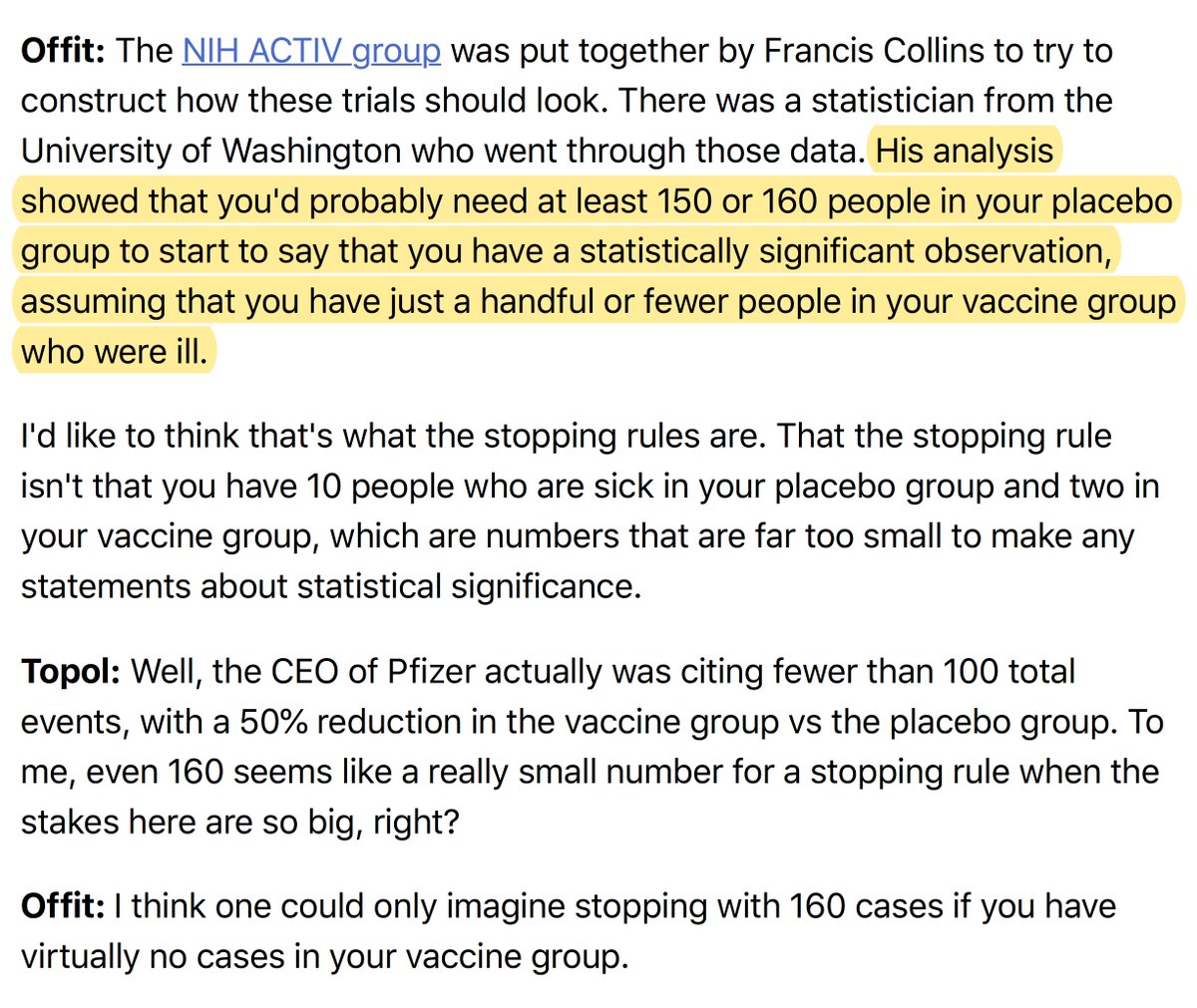
6. How many events does the Data and Safety Monitoring Board need to make the call?
*None of the companies have published their data analysis plans*
*Or their stopping rules for unequivocal efficacy*
150 or 160 total events is what has been transmitted at various conferences
*None of the companies have published their data analysis plans*
*Or their stopping rules for unequivocal efficacy*
150 or 160 total events is what has been transmitted at various conferences
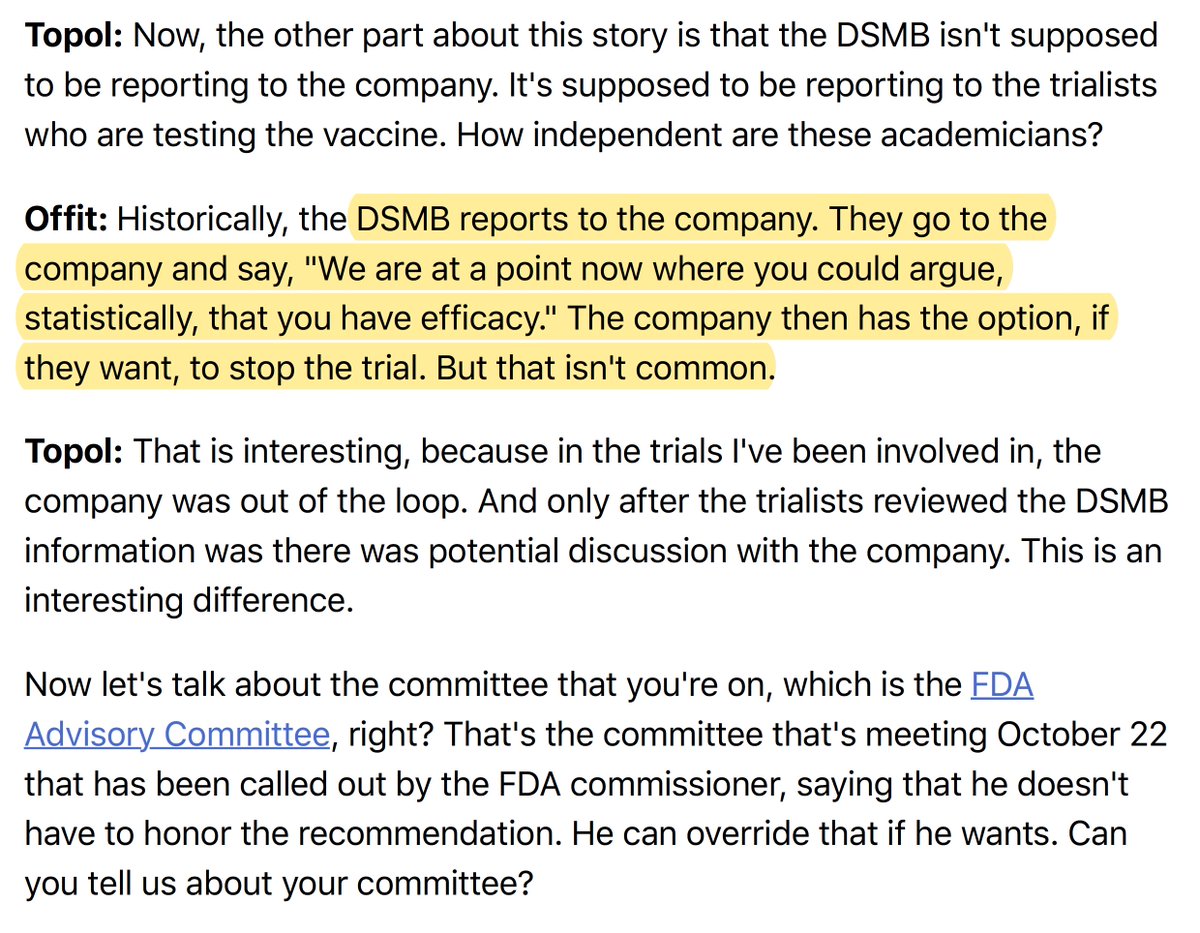
8. My experience is that the DSMB only talks to the clinical trialists and not to the company unless there are safety issues.
What is happening here?
"The DSMB reports to the company"
What is happening here?
"The DSMB reports to the company"
9. What is going to happen at the FDA Advisory Committee on October 22nd that you serve on?
Our committee gives advice. The FDA Commissioner can either take it or not.
Important: Unless the code is broken by the DSMB (to Pfizer or Moderna) we won't see any data at the committee
Our committee gives advice. The FDA Commissioner can either take it or not.
Important: Unless the code is broken by the DSMB (to Pfizer or Moderna) we won't see any data at the committee
10. On safety of vaccines
Concerns about vaccine-induced immunopathology reviewed with prior vaccines including measles, Dengue, RSV
There could be rare events (such as Th1 or Th2 responses) which we'll only know more about when millions of people have been vaccinated
Concerns about vaccine-induced immunopathology reviewed with prior vaccines including measles, Dengue, RSV
There could be rare events (such as Th1 or Th2 responses) which we'll only know more about when millions of people have been vaccinated
11. More on safety
Keep our eyes open regarding people with prior covid exposure
mRNA vaccines have a lipid delivery system
The replication-deficient virus programs give a lot of virus
Adenovirus vector
Keep our eyes open regarding people with prior covid exposure
mRNA vaccines have a lipid delivery system
The replication-deficient virus programs give a lot of virus
Adenovirus vector
12. The trials are not representative for aged, children, underrepresented minorities. We won't know how long efficacy persists
"You have to also make sure people know what you don't know"
"You have to also make sure people know what you don't know"
13. We'll be needing to wear masks for a long time, not just when vaccines are approved or start implementation.
Effective vaccines don't provide mucosal IgA immunity and will increase the rate of asymptomatic carriers
Effective vaccines don't provide mucosal IgA immunity and will increase the rate of asymptomatic carriers
14. Vaccination at scale, hundreds of millions of doses, the need for two-dose vaccines and subsequent boosters, requiring acceptance of the public will be a major challenge

 Read on Twitter
Read on Twitter