2/
The request was granted and in 1924 he decided to thinly cut and mount slices of rat tumor in Ringer solution. He then measured lactic acid (lactate) production.
The request was granted and in 1924 he decided to thinly cut and mount slices of rat tumor in Ringer solution. He then measured lactic acid (lactate) production.
3/
Why? Well in 1863, Pasteur showed that a hypoxic environment induced the fermentation of sugars. Warburg essentially wanted to determine how tumors respire. It would follow that in a hypoxic environment, tumors would produce lactate.
Why? Well in 1863, Pasteur showed that a hypoxic environment induced the fermentation of sugars. Warburg essentially wanted to determine how tumors respire. It would follow that in a hypoxic environment, tumors would produce lactate.
4/
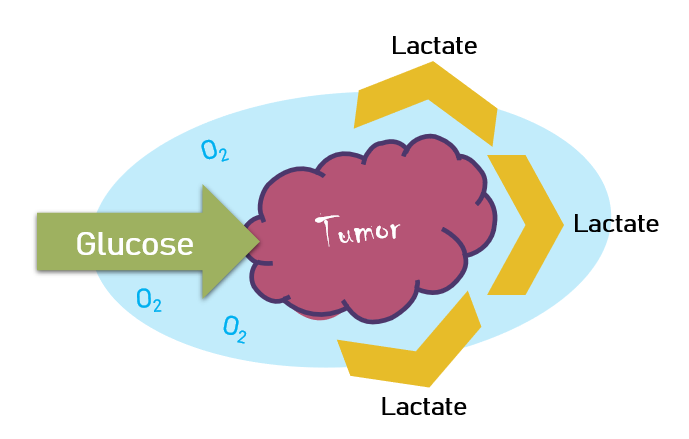
But this was not the case! Warburg noted that even in the presence of oxygen, his tumors produced lactate.
But this was not the case! Warburg noted that even in the presence of oxygen, his tumors produced lactate.
5/
He concluded that a tumor's cellular respiration (TCA/Krebs cycle) was in some way damaged. Although he won the 1931 Nobel Prize in Physiology and Medicine for his discovery of cytochrome c, he is probably more famous for what is now called the 'Warburg Effect.'
He concluded that a tumor's cellular respiration (TCA/Krebs cycle) was in some way damaged. Although he won the 1931 Nobel Prize in Physiology and Medicine for his discovery of cytochrome c, he is probably more famous for what is now called the 'Warburg Effect.'
6/
The Warburg Effect effectively states that tumors preferentially choose glycolysis over aerobic respiration. And this assumption was seemingly verified into the 2000s. Multiple labs linked oncogenes to glycolysis.
The Warburg Effect effectively states that tumors preferentially choose glycolysis over aerobic respiration. And this assumption was seemingly verified into the 2000s. Multiple labs linked oncogenes to glycolysis.
7/
And this switch/change was metabolically viable. Cells can use lactate to re-enter glycolysis. Multiple alternative pathways can generate ATP from excess lactate.
And this switch/change was metabolically viable. Cells can use lactate to re-enter glycolysis. Multiple alternative pathways can generate ATP from excess lactate.
8/
And we know that muscles under strain do this already! Dr. George Brooks has published multiple excellent papers on the "Lactate Shuttle." In normal cells, lactate acts as fuel and as an adaptive 'hormone.' But this signaling stops when exercise stops.
And we know that muscles under strain do this already! Dr. George Brooks has published multiple excellent papers on the "Lactate Shuttle." In normal cells, lactate acts as fuel and as an adaptive 'hormone.' But this signaling stops when exercise stops.
9/
This isn't the case in greedy cancer cells. Unchecked increases in lactate production lead to maladaptive changes. The host bears the burden of the tumor's glucose needs and acts as a waste disposal site for lactate ions.
This isn't the case in greedy cancer cells. Unchecked increases in lactate production lead to maladaptive changes. The host bears the burden of the tumor's glucose needs and acts as a waste disposal site for lactate ions.
10/
We've used this hungry tumor paradigm for PET/CTs. Hot tumors are those that are radiolabeled with 18F-FDG, a glucose conjugate.
We've used this hungry tumor paradigm for PET/CTs. Hot tumors are those that are radiolabeled with 18F-FDG, a glucose conjugate.
11/
So why is a pathologist posting all this biochemical stuff about lactate and glucose? Well, I was partially inspired by a post I made https://twitter.com/Dr_Brian_Cox/status/1291440947310419968
So why is a pathologist posting all this biochemical stuff about lactate and glucose? Well, I was partially inspired by a post I made https://twitter.com/Dr_Brian_Cox/status/1291440947310419968
13/
This led me to a Nature Metabolism paper from Dr. DeBerardinis ( @RJDLab) and Dr. Chandel titled: "We Need to Talk About the Warburg Effect." https://doi.org/10.1038/s42255-020-0172-2
This led me to a Nature Metabolism paper from Dr. DeBerardinis ( @RJDLab) and Dr. Chandel titled: "We Need to Talk About the Warburg Effect." https://doi.org/10.1038/s42255-020-0172-2
14/
Where they state: "...genetically defined impairments in oxidative metabolism may stimulate aerobic glycolysis in cancer, but, in general, aerobic glycolysis does not predict loss of oxidative metabolism."
Where they state: "...genetically defined impairments in oxidative metabolism may stimulate aerobic glycolysis in cancer, but, in general, aerobic glycolysis does not predict loss of oxidative metabolism."
15/
Essentially, tumors and tumor syndromes (below) associated with mutations of the TCA cycle are both Warburgian and un-Warburgian. Mutations in SDH, for instance, damage cellular respiration, but this is an 'a priori' event. It is not the result of some oncogene mutation.
Essentially, tumors and tumor syndromes (below) associated with mutations of the TCA cycle are both Warburgian and un-Warburgian. Mutations in SDH, for instance, damage cellular respiration, but this is an 'a priori' event. It is not the result of some oncogene mutation.
16/
Thank you for joining me on this rabbit hole, I hope you found it interesting and possibly learned something! I will be using a larger version of this for my Grand Rounds in September, so any clarifications or new insights are welcome. Below are my references :)
Thank you for joining me on this rabbit hole, I hope you found it interesting and possibly learned something! I will be using a larger version of this for my Grand Rounds in September, so any clarifications or new insights are welcome. Below are my references :)
17/
#tweetorial #warburgeffect #lactateshuttle #SDH #glycolysis #carcinoma #tumor #premedtwitter #medtwitter #pathtwitter #pathology #metabolism #cancermetabolism #oncology
#tweetorial #warburgeffect #lactateshuttle #SDH #glycolysis #carcinoma #tumor #premedtwitter #medtwitter #pathtwitter #pathology #metabolism #cancermetabolism #oncology

 Read on Twitter
Read on Twitter