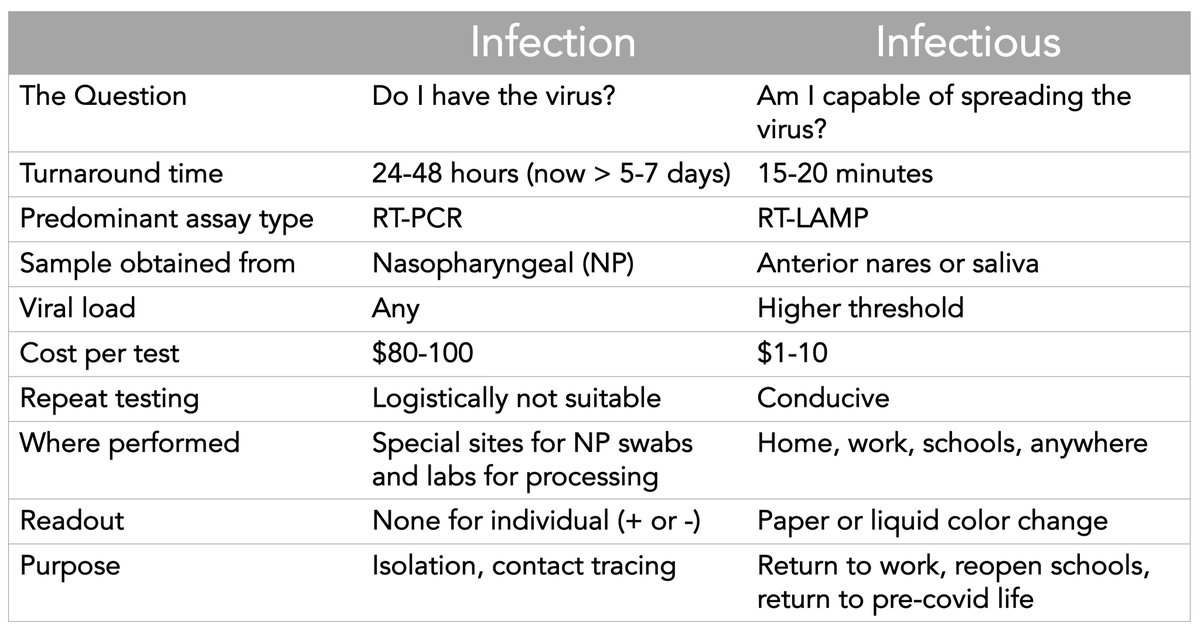
The time to move to rapid #SARSCoV2 testing is long overdue. It's about switching from diagnosing *infections* to determining whether someone is *infectious*
In minutes, not days. Anywhere. Cheap.
My table here summarizes the differences and why this should be the #1 US priority
In minutes, not days. Anywhere. Cheap.
My table here summarizes the differences and why this should be the #1 US priority
In order to get this done, we need a reboot at @US_FDA, which currently requires rapid tests to perform like PCR tests.
That's wrong. This is a new diagnostic category for the *infectious* endpoint, requiring new standards and prospective validation.
That's wrong. This is a new diagnostic category for the *infectious* endpoint, requiring new standards and prospective validation.
The @US_FDA just came around today (yes, a new template, a coincidence with the prior post). Great to see a sign of progress
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-posts-new-template-home-and-over-counter-diagnostic-tests-use-non via @nataliexdean @DanLarremore
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-posts-new-template-home-and-over-counter-diagnostic-tests-use-non via @nataliexdean @DanLarremore

 Read on Twitter
Read on Twitter