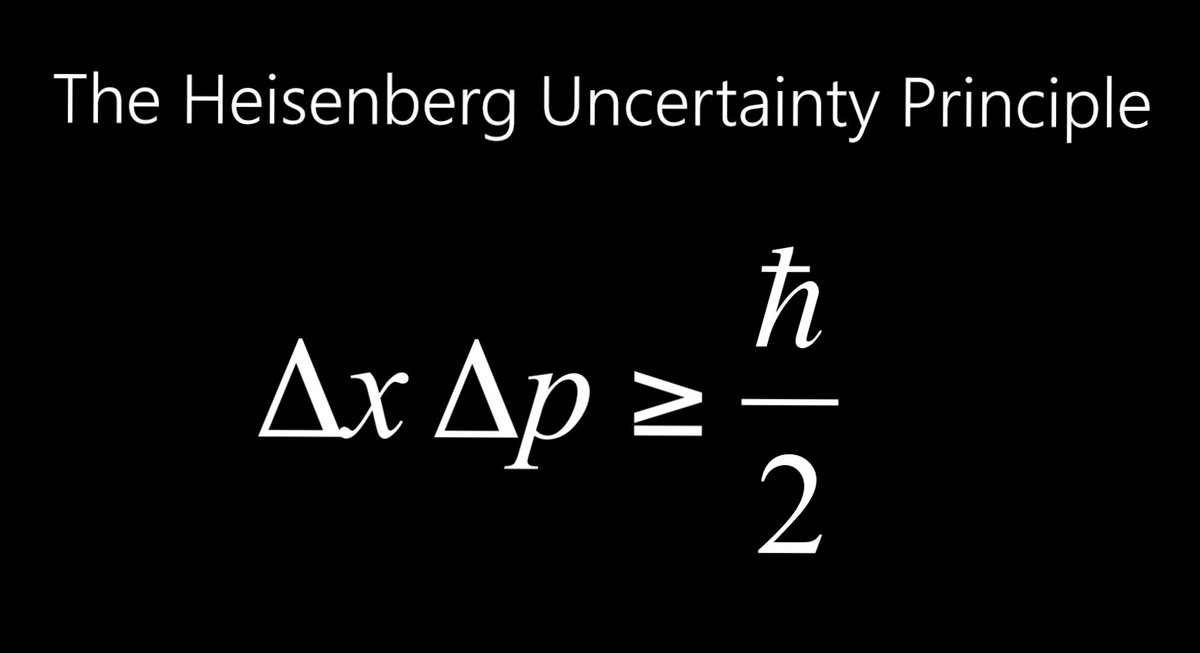Heisenberg's Uncertainty Principle (a thread):
In Feb 23, 1927, physicist Werner Heisenberg outlined his new principle in a 14-page letter to Wolfgang Pauli. In March, he submitted his paper for publication. Niels Bohr pointed out some errors, but agreed that the principle..
In Feb 23, 1927, physicist Werner Heisenberg outlined his new principle in a 14-page letter to Wolfgang Pauli. In March, he submitted his paper for publication. Niels Bohr pointed out some errors, but agreed that the principle..
itself was correct and the paper was published. The paper was titled "On the Perceptual Content of Quantum Theoretical Kinematics and Mechanics." The more familiar form of the eqn came a few years later when he had further refined his thoughts in subsequent lectures and papers.
The new principle had deep implications. The principle soon became part of the basis for the Copenhagen interpretation of QM, and at the Solvay conference that fall, Heisenberg and Max Born declared the quantum revolution complete.
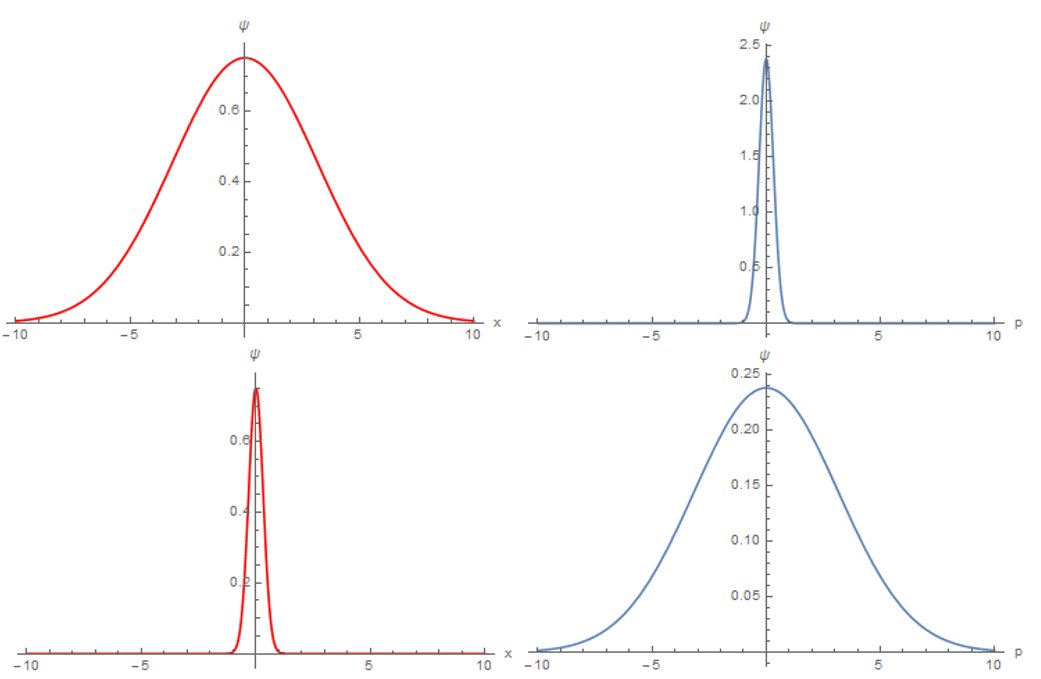
Heisenberg's Uncertainty Principle states that: The more accurately we know a particle's position, the less accurately we can know its momentum. Both the position and momentum of a particle can not be determined precisely at a given time.
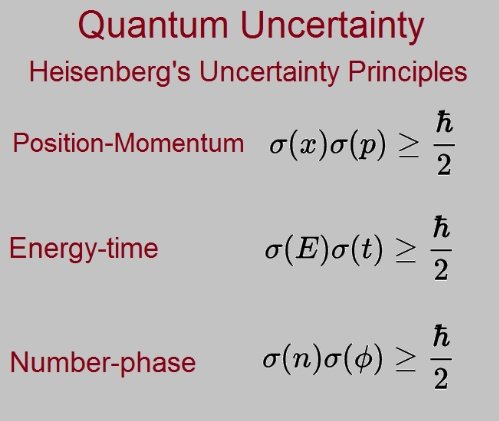
Mathematically, the Heisenberg uncertainty principle is a lower bound on the product of uncertainties of a pair of conjugate variables. The most well-known expression takes the position and momentum to be the conjugate variables:
Another uncertainty relation which is often referenced in discussion of quantum mechanics is the energy-time uncertainty principle,
It is tempting to interpret this equation as the statement that a system may fluctuate in energy by an arbitrarily large amount over a sufficiently short time scale. The principle is at the heart of many things that we observe but cannot explain using classical physics.
Take atoms, for example, where negatively-charged electrons orbit a positively-charged nucleus. We might expect, by classical logic, the two opposite charges to attract each other, leading everything to collapse into a ball of particles.
The uncertainty principle explains why this doesn't happen: if an electron got too close to the nucleus, then its position in space would be precisely known and, therefore, the error in measuring its position would be minuscule.
This means that the error in measuring its momentum (and, by inference, its velocity) would be enormous. In that case, the electron could be moving fast enough to fly out of the atom altogether.
Heisenberg's idea can also explain a type of nuclear radiation called alpha decay. Perhaps the strangest result of the uncertainty principle is what it explains about the absence of particles in vacuum.
Uncertainty, then, is nothing to worry about in quantum physics and, in fact, we wouldn't be here if this principle didn't exist. It has wide range of implications in our day to day lives and the world around us.
Follow @PhysInHistory for more fascinating facts and photos from the history of physics. 


 Read on Twitter
Read on Twitter