Excited to share the results of @SWOG 1500, which we are presenting at @ASCO #GU21 & reporting in @TheLancet today. 1st, THANK YOU to patients & families. 2nd, hats off to a tremendous group of investigators. This was a team effort! Wanted to share how it all began: (1/13)
A telling snapshot from my "S1500 folder" on my computer. With mentorship from @PrimoLaraMD I submitted the concept 1st in 2012. Had advice from my mentor @DrChoueiri at the time as I conceived of the study design - interestingly, the proposal was XL184 (cabo) v sunitinib! (2/13)
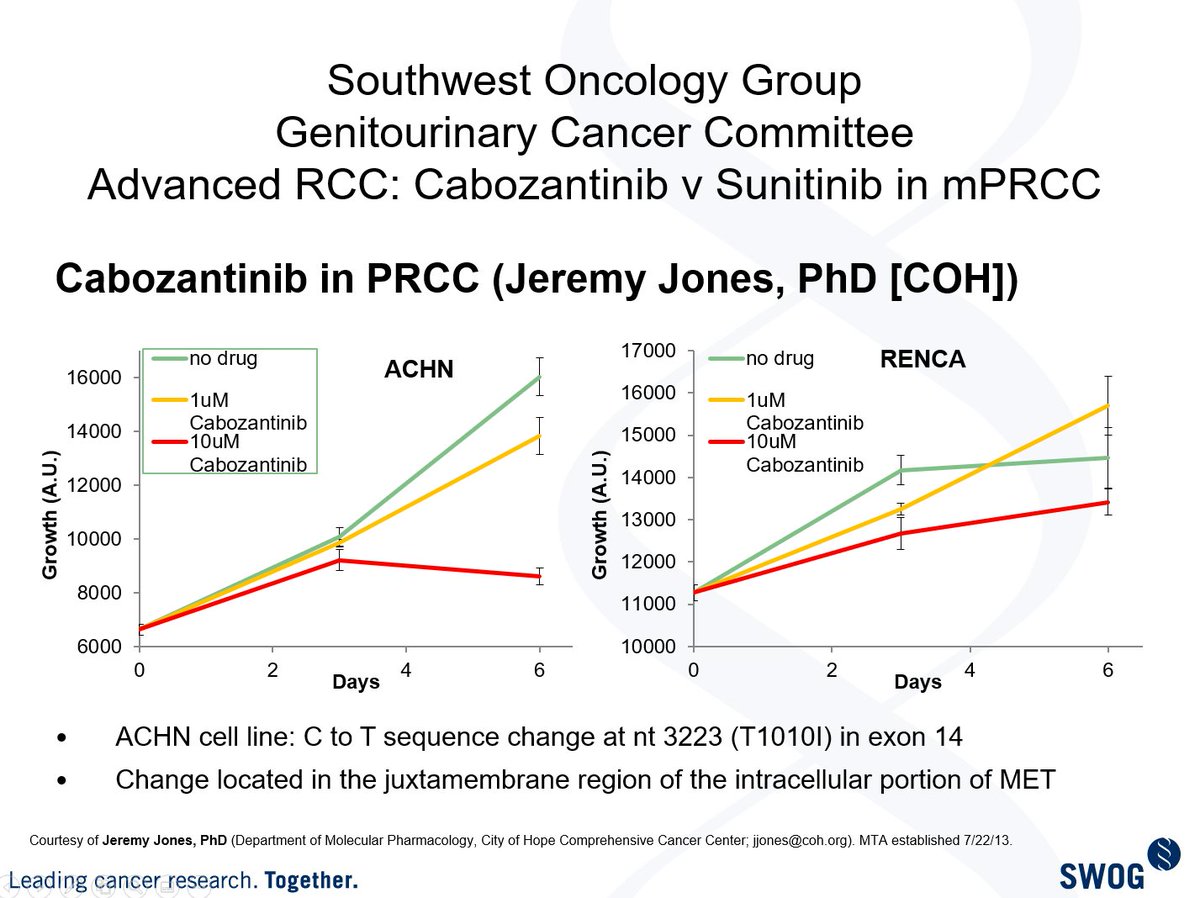
My dear friend @Jeremy_O_Jones helped me w some preclin work & with emerging evidence from the P1 trial that @DrChoueiri #DrMcDermott #DrJanDutcher & I (along with others), we proposed the following formal design to the group in 2013: (3/13)
That's when things got interesting. @PrimoLaraMD @KidneyCancerDoc & I started meeting weekly w #DrJohnWright @NCICTEP_ClinRes #DrNaomiHaas @PennCancer @drmnstein @ColumbiaUrology #DrCathyTangen @SWOG. Our study expanded: (4/13)
Obvious this made things tricky, both from a statistical & practical standpoint. Some companies pulled out & we saw encouraging results from @DrChoueiri's savolitinib study in @ASCO_pubs #JCO, so we pulled that in: (5/13)
Here is the timeline for emphasis. Have to confess, there were many times when I wasn't sure the trial was going to happen. I'm sure others with cooperative group experience would agree ( @TiansterZhang @DrChoueiri @DrVaishampayan). But we pushed forward: (6/13)
How did we get this done? By getting a little help from our friends. We got @DrDanielHeng @CDNCancerTrials & @Daniel_J_George @TiansterZhang @ALLIANCE_org & MOST IMPORTANTLY fantastic pt advocates like @PeggyZuckerman #RickBangs @RCCadvocate et al: (7/13)
Even once we got things started, it was not smooth sailing. You can see here that accrual was slow. We averaged 3.6 pts/mo. #SAVOIR, presented by @DrChoueiri at #ASCO20, accrued faster despite being a genomically driven trial! Again, advocates played a key role: (8/13)
Ultimately, the joint efforts of @SWOG @ALLIANCE_org @eaonc @NRGonc @CDNCancerTrials & top accruers @cityofhope @PennCancer @karmanoscancer @Sunnybrook pushed this across the finish line. Note that 54 sites put 1 patient on. This contribution was essential as well! (9/13)
So why this lengthy post that focuses nothing on the data? Well, I wanted to encourage brilliant young investigators like @ShuchiGulati @PBarataMD @AbhiTrip87 et al to be persistent in @theNCI cooperative groups. With time, your studies will get done! (10/13)
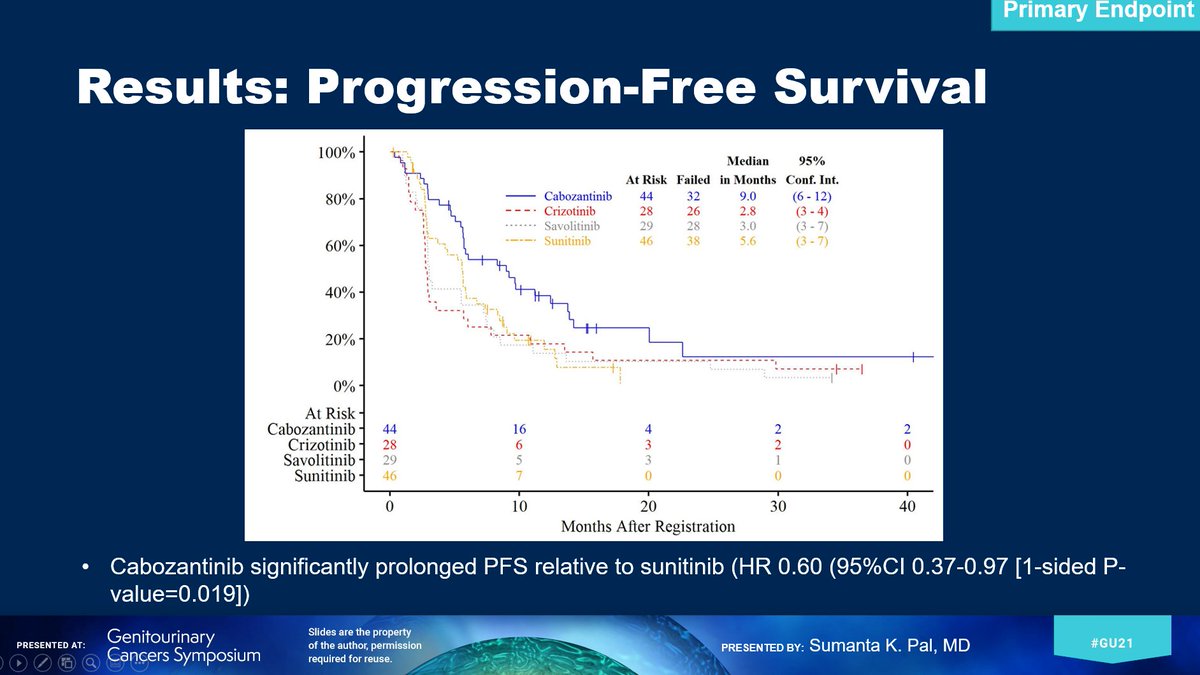
Now a quick work on the data, which you can see in detail in @TheLancet. Cabo is no doubt the standard for now for metastatic papillary #kidneycancer. Having said that, patients still need more! https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00152-5/fulltext (11/13)
I hope we can see more innovative, genomically driven studies like the #SAVOIR study led by @DrChoueiri @DanaFarberNews - wish that study had continued, there was a real signal there! Also see compelling data from #CALYPSO from @tompowles1 #DrCristinaSuarez (12/13)
Finally, REALLY hoping @maughanonc & I can push forward a study we have proposed through @SWOG evaluating cabo +/- IO, which he developed at @SupportingSWOG YITC (a formative course I attended 9 years ago!). Let's push forward for our patients! (13/13)

 Read on Twitter
Read on Twitter