#Tweetorial1️
 Only ~40-60% of GEA pts receive 2L Rx in the USA, & the treatment landscape is fragmented.
Only ~40-60% of GEA pts receive 2L Rx in the USA, & the treatment landscape is fragmented.
 https://pubmed.ncbi.nlm.nih.gov/25792290/
https://pubmed.ncbi.nlm.nih.gov/25792290/
 https://pubmed.ncbi.nlm.nih.gov/31056940/
https://pubmed.ncbi.nlm.nih.gov/31056940/
@TumorBoardTues #TumorBoardTuesday
 Only ~40-60% of GEA pts receive 2L Rx in the USA, & the treatment landscape is fragmented.
Only ~40-60% of GEA pts receive 2L Rx in the USA, & the treatment landscape is fragmented. https://pubmed.ncbi.nlm.nih.gov/25792290/
https://pubmed.ncbi.nlm.nih.gov/25792290/  https://pubmed.ncbi.nlm.nih.gov/31056940/
https://pubmed.ncbi.nlm.nih.gov/31056940/ @TumorBoardTues #TumorBoardTuesday
#Tweetorial2
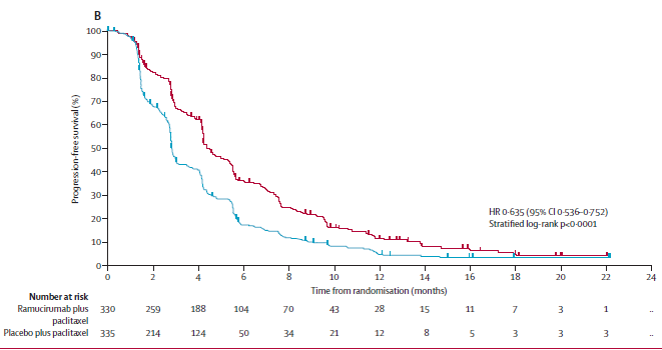
 The Phase III 2L RAINBOW study of paclitaxel-Ram vs Paclitaxel had improved OS, serving as a benchmark SOC
The Phase III 2L RAINBOW study of paclitaxel-Ram vs Paclitaxel had improved OS, serving as a benchmark SOC
 https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(14)70420-6/fulltext
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(14)70420-6/fulltext
 The Phase III 2L RAINBOW study of paclitaxel-Ram vs Paclitaxel had improved OS, serving as a benchmark SOC
The Phase III 2L RAINBOW study of paclitaxel-Ram vs Paclitaxel had improved OS, serving as a benchmark SOC https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(14)70420-6/fulltext
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(14)70420-6/fulltext
#Tweetorial3
 The Phase III 2L KN061 study showed detriment for pts with PDL1 CPS 0 treated w pembro vs paclitaxel (no Ram!), and terminated this group early.
The Phase III 2L KN061 study showed detriment for pts with PDL1 CPS 0 treated w pembro vs paclitaxel (no Ram!), and terminated this group early.
(green = pembro, red = paclitaxel)
 https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31257-1/fulltext
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31257-1/fulltext
 https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31257-1/fulltext#supplementaryMaterial
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31257-1/fulltext#supplementaryMaterial
 The Phase III 2L KN061 study showed detriment for pts with PDL1 CPS 0 treated w pembro vs paclitaxel (no Ram!), and terminated this group early.
The Phase III 2L KN061 study showed detriment for pts with PDL1 CPS 0 treated w pembro vs paclitaxel (no Ram!), and terminated this group early. (green = pembro, red = paclitaxel)
 https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31257-1/fulltext
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31257-1/fulltext  https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31257-1/fulltext#supplementaryMaterial
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31257-1/fulltext#supplementaryMaterial
#Tweetorial4
 Studies show that FOLFIRI is an option vs paclitaxel, along w ramucirumab, in certain situations-
Studies show that FOLFIRI is an option vs paclitaxel, along w ramucirumab, in certain situations-
1) persistent oxaliplatin neuropathy or
2) previous Rx with platinum/taxane perioperatively or 1L:
 https://pubmed.ncbi.nlm.nih.gov/30470690/
https://pubmed.ncbi.nlm.nih.gov/30470690/
 https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.4514
https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.4514
 Studies show that FOLFIRI is an option vs paclitaxel, along w ramucirumab, in certain situations-
Studies show that FOLFIRI is an option vs paclitaxel, along w ramucirumab, in certain situations- 1) persistent oxaliplatin neuropathy or
2) previous Rx with platinum/taxane perioperatively or 1L:
 https://pubmed.ncbi.nlm.nih.gov/30470690/
https://pubmed.ncbi.nlm.nih.gov/30470690/  https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.4514
https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.4514
#Tweetorial5a
 2L studies were -ve for anti-HER2 Rx in 2L:
2L studies were -ve for anti-HER2 Rx in 2L:
 Phase 3 TyTAN (lapatinib) https://pubmed.ncbi.nlm.nih.gov/24868024/
Phase 3 TyTAN (lapatinib) https://pubmed.ncbi.nlm.nih.gov/24868024/
 Phase 3 GATSBY (TDM1) https://pubmed.ncbi.nlm.nih.gov/28343975/
Phase 3 GATSBY (TDM1) https://pubmed.ncbi.nlm.nih.gov/28343975/
 Phase 2 T-ACT (trastuzumab) https://pubmed.ncbi.nlm.nih.gov/32208960/
Phase 2 T-ACT (trastuzumab) https://pubmed.ncbi.nlm.nih.gov/32208960/
 2L studies were -ve for anti-HER2 Rx in 2L:
2L studies were -ve for anti-HER2 Rx in 2L: Phase 3 TyTAN (lapatinib) https://pubmed.ncbi.nlm.nih.gov/24868024/
Phase 3 TyTAN (lapatinib) https://pubmed.ncbi.nlm.nih.gov/24868024/  Phase 3 GATSBY (TDM1) https://pubmed.ncbi.nlm.nih.gov/28343975/
Phase 3 GATSBY (TDM1) https://pubmed.ncbi.nlm.nih.gov/28343975/  Phase 2 T-ACT (trastuzumab) https://pubmed.ncbi.nlm.nih.gov/32208960/
Phase 2 T-ACT (trastuzumab) https://pubmed.ncbi.nlm.nih.gov/32208960/
#Tweetorial5b
Some contributing reasons for these -ve studies include:
1) Allowed HER2 IHC0/1+ w FISH+ (based on pre-1L profile) (TyTAN = (35%!))
2) Not rechecking HER2 status after PD on 1L Rx (all 3 studies)
 A subgroup of T-ACT tumors at just prior to 2L 69% (!) were HER2-
A subgroup of T-ACT tumors at just prior to 2L 69% (!) were HER2-
Some contributing reasons for these -ve studies include:
1) Allowed HER2 IHC0/1+ w FISH+ (based on pre-1L profile) (TyTAN = (35%!))
2) Not rechecking HER2 status after PD on 1L Rx (all 3 studies)
 A subgroup of T-ACT tumors at just prior to 2L 69% (!) were HER2-
A subgroup of T-ACT tumors at just prior to 2L 69% (!) were HER2-
#Tweetorial5c
3) Relatively small studies w power
power
 Despite this, in TyTAN, ORR ITT was 27% vs 9% in favor of pac/lap vs pac,
Despite this, in TyTAN, ORR ITT was 27% vs 9% in favor of pac/lap vs pac,
 & in those with pre-1L IHC3+ (who r less likely to evolve to neg @ 2L) --> OS advantage (ORR not reported but likely higher: 40%?, 50%?):
& in those with pre-1L IHC3+ (who r less likely to evolve to neg @ 2L) --> OS advantage (ORR not reported but likely higher: 40%?, 50%?):
3) Relatively small studies w
 power
power  Despite this, in TyTAN, ORR ITT was 27% vs 9% in favor of pac/lap vs pac,
Despite this, in TyTAN, ORR ITT was 27% vs 9% in favor of pac/lap vs pac,  & in those with pre-1L IHC3+ (who r less likely to evolve to neg @ 2L) --> OS advantage (ORR not reported but likely higher: 40%?, 50%?):
& in those with pre-1L IHC3+ (who r less likely to evolve to neg @ 2L) --> OS advantage (ORR not reported but likely higher: 40%?, 50%?):
#Tweetorial6️
 Recent papers evaluating HER2 conversion show rates of conversion between 15-45%.
Recent papers evaluating HER2 conversion show rates of conversion between 15-45%.
 The longer the pt is on & over more lines of anti-HER2 Rx may increase this finding.
The longer the pt is on & over more lines of anti-HER2 Rx may increase this finding.
 https://pubmed.ncbi.nlm.nih.gov/29122777/
https://pubmed.ncbi.nlm.nih.gov/29122777/
 https://pubmed.ncbi.nlm.nih.gov/33234578/
https://pubmed.ncbi.nlm.nih.gov/33234578/
 Recent papers evaluating HER2 conversion show rates of conversion between 15-45%.
Recent papers evaluating HER2 conversion show rates of conversion between 15-45%.  The longer the pt is on & over more lines of anti-HER2 Rx may increase this finding.
The longer the pt is on & over more lines of anti-HER2 Rx may increase this finding.  https://pubmed.ncbi.nlm.nih.gov/29122777/
https://pubmed.ncbi.nlm.nih.gov/29122777/  https://pubmed.ncbi.nlm.nih.gov/33234578/
https://pubmed.ncbi.nlm.nih.gov/33234578/
#Tweetorial7
 Tras-Derux in Asian phase 2 in 3L+ (~55% 3L, 45% 4L+) showed better ORR, PFS, and OS compared to MD choicetaxane/irinotecan.
Tras-Derux in Asian phase 2 in 3L+ (~55% 3L, 45% 4L+) showed better ORR, PFS, and OS compared to MD choicetaxane/irinotecan.
 https://www.nejm.org/doi/full/10.1056/NEJMoa2004413
https://www.nejm.org/doi/full/10.1056/NEJMoa2004413
 Approved in Japan for 3L+ 9/25/20
Approved in Japan for 3L+ 9/25/20
 Approved by FDA for 2L+
Approved by FDA for 2L+  1/15/21.
1/15/21.
Black Box: ILD, Reassess HER2 bx!
 Tras-Derux in Asian phase 2 in 3L+ (~55% 3L, 45% 4L+) showed better ORR, PFS, and OS compared to MD choicetaxane/irinotecan.
Tras-Derux in Asian phase 2 in 3L+ (~55% 3L, 45% 4L+) showed better ORR, PFS, and OS compared to MD choicetaxane/irinotecan.  https://www.nejm.org/doi/full/10.1056/NEJMoa2004413
https://www.nejm.org/doi/full/10.1056/NEJMoa2004413  Approved in Japan for 3L+ 9/25/20
Approved in Japan for 3L+ 9/25/20 Approved by FDA for 2L+
Approved by FDA for 2L+  1/15/21.
1/15/21. Black Box: ILD, Reassess HER2 bx!
#Tweetorial8a Case Discussion 1/2
 CPS 0 (no pembro), per #KN061
CPS 0 (no pembro), per #KN061
 ctDNA HER2 -ve (despite PT HER2+) predicts likely failure here systemically. I would use Chemo+Ram as their next best 2L option.
ctDNA HER2 -ve (despite PT HER2+) predicts likely failure here systemically. I would use Chemo+Ram as their next best 2L option.
(Welcome comments on this point - let's debate! )
)
 CPS 0 (no pembro), per #KN061
CPS 0 (no pembro), per #KN061 ctDNA HER2 -ve (despite PT HER2+) predicts likely failure here systemically. I would use Chemo+Ram as their next best 2L option.
ctDNA HER2 -ve (despite PT HER2+) predicts likely failure here systemically. I would use Chemo+Ram as their next best 2L option.(Welcome comments on this point - let's debate!
 )
)
#Tweetorial8b Case Discussion 2/2
 tumor biopsies can be useful for reassessing tumor biology:
tumor biopsies can be useful for reassessing tumor biology:
 https://pubmed.ncbi.nlm.nih.gov/33234578/
https://pubmed.ncbi.nlm.nih.gov/33234578/
 but so can liquid biopsies be useful for reassessing tumor biology, and may better represent the dz in its entirety:
but so can liquid biopsies be useful for reassessing tumor biology, and may better represent the dz in its entirety:
 https://pubmed.ncbi.nlm.nih.gov/31427281/
https://pubmed.ncbi.nlm.nih.gov/31427281/
 tumor biopsies can be useful for reassessing tumor biology:
tumor biopsies can be useful for reassessing tumor biology: https://pubmed.ncbi.nlm.nih.gov/33234578/
https://pubmed.ncbi.nlm.nih.gov/33234578/  but so can liquid biopsies be useful for reassessing tumor biology, and may better represent the dz in its entirety:
but so can liquid biopsies be useful for reassessing tumor biology, and may better represent the dz in its entirety: https://pubmed.ncbi.nlm.nih.gov/31427281/
https://pubmed.ncbi.nlm.nih.gov/31427281/
#Tweetorial9a
 For persistently HER2+ tumors, phase 3 2L studies are ongoing, both requiring retesting to confirm HER2+:
For persistently HER2+ tumors, phase 3 2L studies are ongoing, both requiring retesting to confirm HER2+:
 Pac/Ram vs Pac/Ram/Tucatinib/Trastuzumab https://clinicaltrials.gov/ct2/show/NCT04499924
Pac/Ram vs Pac/Ram/Tucatinib/Trastuzumab https://clinicaltrials.gov/ct2/show/NCT04499924
 Pac/Ram vs T-DXd https://www.clinicaltrials.gov/ct2/show/NCT04704934?term=DESTINY-Gastric04&draw=2&rank=1
Pac/Ram vs T-DXd https://www.clinicaltrials.gov/ct2/show/NCT04704934?term=DESTINY-Gastric04&draw=2&rank=1
 For persistently HER2+ tumors, phase 3 2L studies are ongoing, both requiring retesting to confirm HER2+:
For persistently HER2+ tumors, phase 3 2L studies are ongoing, both requiring retesting to confirm HER2+: Pac/Ram vs Pac/Ram/Tucatinib/Trastuzumab https://clinicaltrials.gov/ct2/show/NCT04499924
Pac/Ram vs Pac/Ram/Tucatinib/Trastuzumab https://clinicaltrials.gov/ct2/show/NCT04499924  Pac/Ram vs T-DXd https://www.clinicaltrials.gov/ct2/show/NCT04704934?term=DESTINY-Gastric04&draw=2&rank=1
Pac/Ram vs T-DXd https://www.clinicaltrials.gov/ct2/show/NCT04704934?term=DESTINY-Gastric04&draw=2&rank=1
#Tweetorial9b
*My Opinion: With 10% ILD risk, moving T’Dxd earlier than 3L should require direct large-scale comparison to standard Pac/Ram and most importantly, to other SOC (readily available) as well as investigational anti-HER2 approaches.
Cheers and Happy Valentine's
and Happy Valentine's
*My Opinion: With 10% ILD risk, moving T’Dxd earlier than 3L should require direct large-scale comparison to standard Pac/Ram and most importantly, to other SOC (readily available) as well as investigational anti-HER2 approaches.

Cheers
 and Happy Valentine's
and Happy Valentine's
SUMMARY:
#targetedtherapiesfortargetedpopulations
Treating with the right drug, at the right dose, at the right time. #MedTwitter #TumorBoardTuesday
#MedTwitter #TumorBoardTuesday
#targetedtherapiesfortargetedpopulations
Treating with the right drug, at the right dose, at the right time.
 #MedTwitter #TumorBoardTuesday
#MedTwitter #TumorBoardTuesday

 Read on Twitter
Read on Twitter