Delighted to share a new study from our lab on the role of gut commensal fungi mycobiota in shaping the human protective IgG antibody repertoire @CellCellPress https://www.cell.com/cell/fulltext/S0092-8674(21)00059-3
During immunosuppression ( organ #Transplant #chemotherapy ) or in diseases such as #IBD #crohnsdisease, gut #mycobiota can turn into a “reservoir” of pathogens, BUT most people live peacefully with their fungi. Do they benefit from them? Do fungi benefit form us? Neutral rl.?
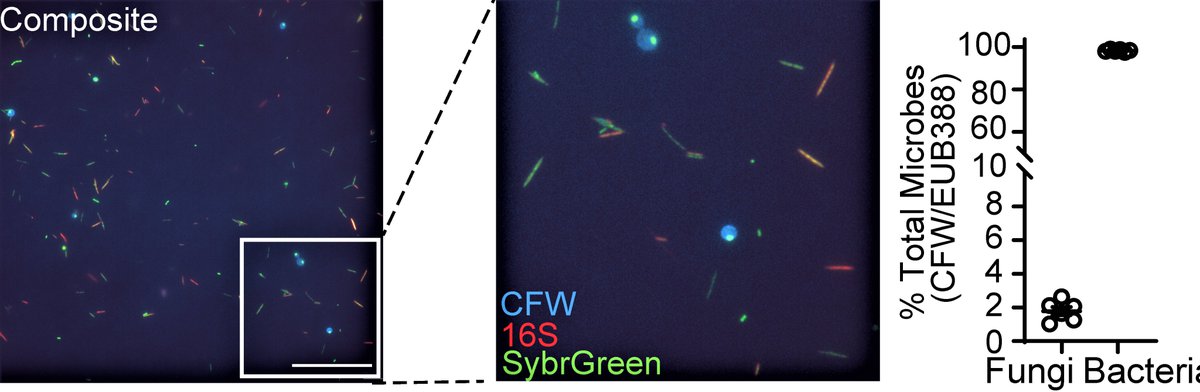
Fungi are considered a minor component of the gut #microbiome ( less than 0.1% of genetic material in feces), but we found that fungal biomass in the healthy human gut makes considerably more: up to 2%.
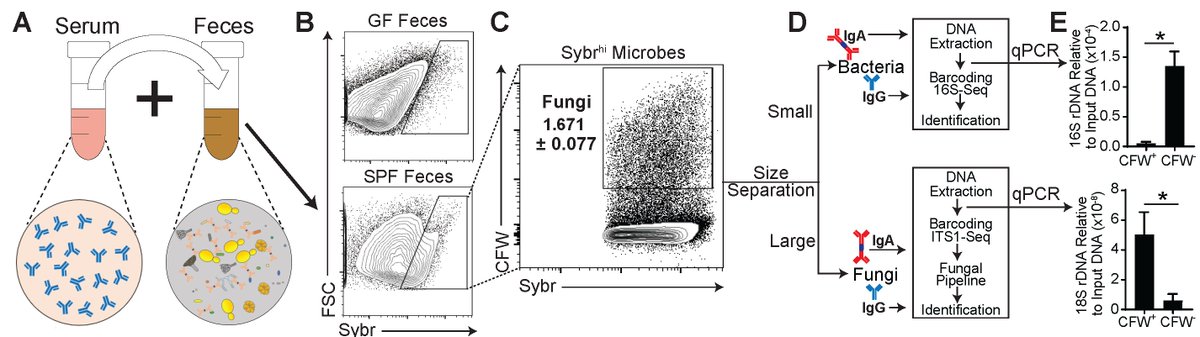
We applied a multi-kingdom antibody profiling or “multiKAP”, designed to amplify microbial signals and distinguish immunoreactive fungi from the rest.
Let me introduce Itai Doron, the first graduate student joining the lab when all this started and a leading force of the study. Big kudos to Itai for his dedication, talent, and persistence. Thank you for taking the leap & joining a lab of a #newPI
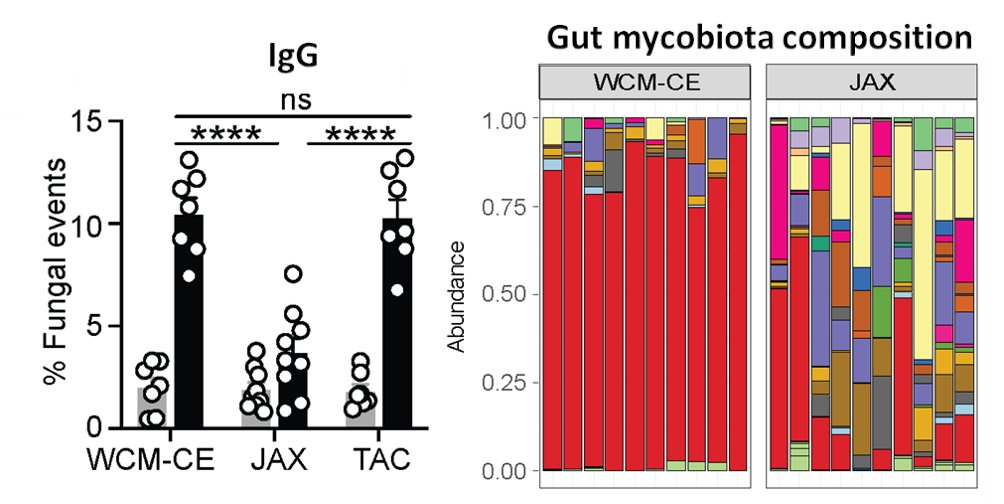
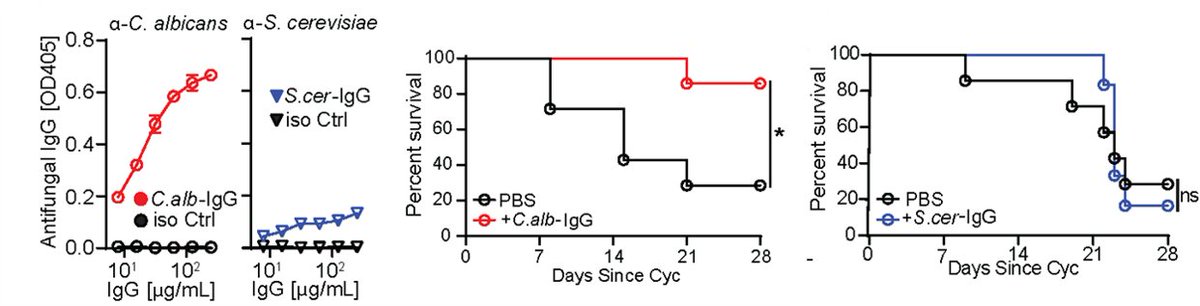
Antibody binding to microbiota isn’t new & we found fungi bound by gut secretory IgA, but a ~20% of the gut mycobiota was recognized by systemic IgG Abs in the blood of healthy humans & in mice. What facility mice come from defined their aF-IgG titers & mycobiota composition.
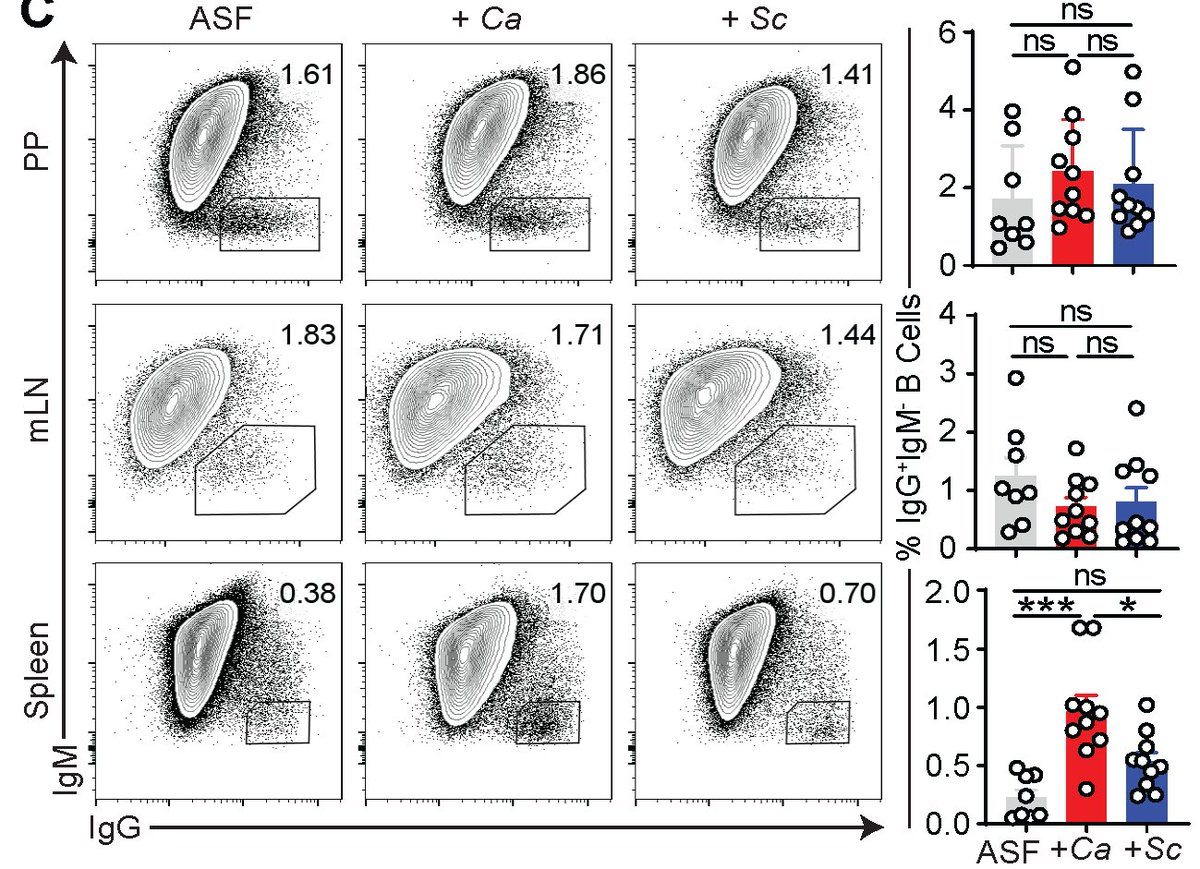
What is the function of these aF-IgG Abs and how they arise? We discovered that systemic aF-IgG Abs arise against specific gut fungi, such as C. albicans & are generated at gut-distal body sites such as spleens. There, they induced IgG B-cell CSR, GC-B cell expansion and diff.
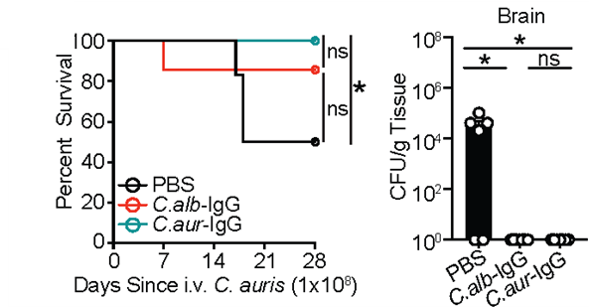
Immunosuppressed patients are at risk of deadly fungal infections, with fungi often coming from the gut. GF mice lack anti-commensal IgG, so we used them to generate aF-IgG antibodies. a-Candida Abs, protected immunosuppressed mice against gut-disseminated systemic candidiasis.
Similar approach protected against emerging multidrug-resistant Candida auris recently flagged by @CDCgov , demonstrating powerful protection by aF-antibodies in the face of broad host cellular immunity depression.
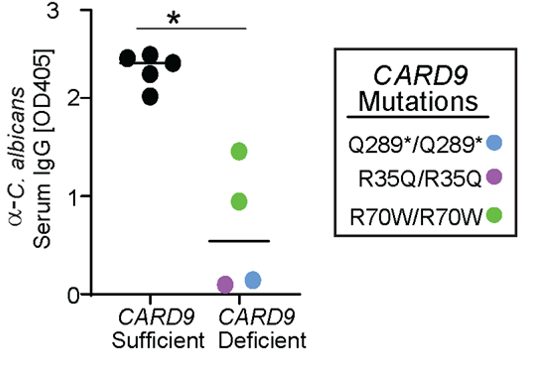
CARD9 deficient patients develop severe fungal infections. We collaborated with Dr. Anne Puel @Inserm who studies these patients. We found CARD9 deficient patients had low titers of anti-C. albicans IgG Abs despite ongoing candidiasis, causingaF-Ab responses in control patients
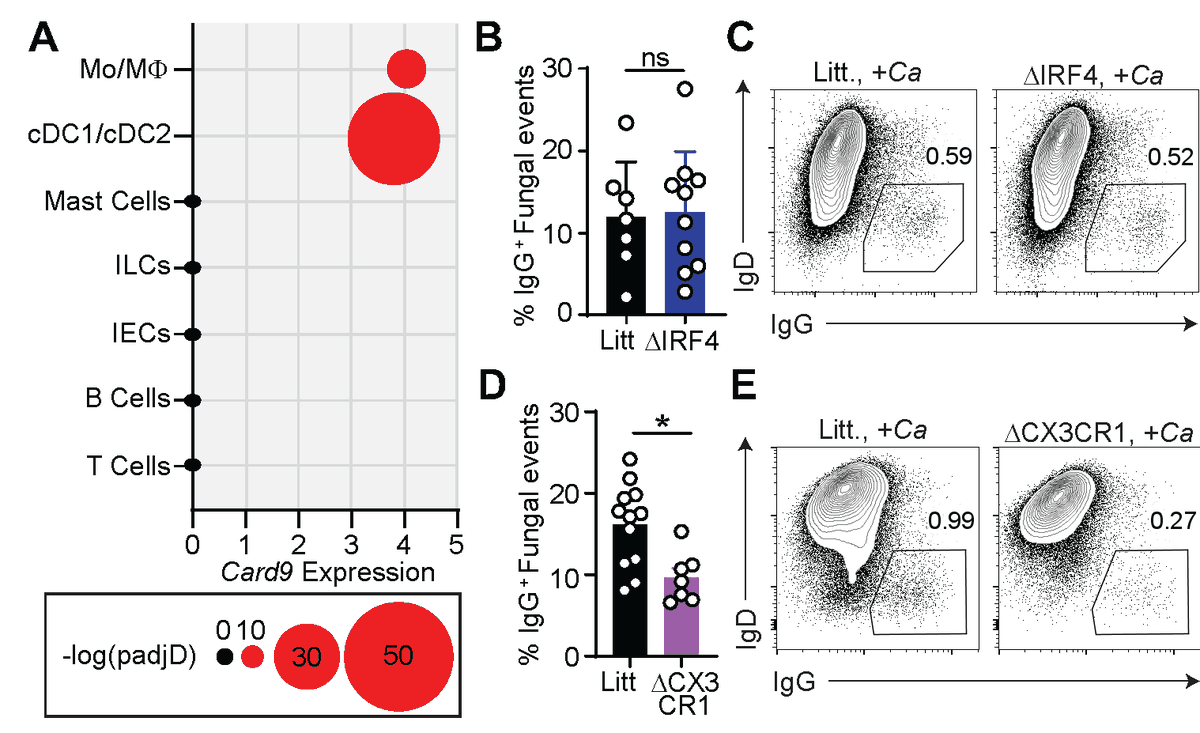
This data & further studies in mice implicated CARD9 (through CARD9+CX3CR1+ macrophages) as a critical mediator of systemic antifungal IgG antibody responses.
This study took 4.5 years. We thank many wonderful collaborators @WeillCornell , @Inserm , @NIH , @KR_Foundation , @CrohnsColitisFn , and new partners @HelmsleyTrust & @BWFUND on exciting new fungal endeavors in #crohnsdisease #allergies & #infectiousdiseases .
And #NYC thank you for all the #bagels & inclusive culture. The #pandemic didn’t help with experiments, but bound us even more (new picture hopefully soon)

 Read on Twitter
Read on Twitter