@JNJNews has officially submitted for emergency use authorization with the FDA. Last week we got the results for its Phase 3 Covid-19 vaccine trial. Here’s a thread on what we know about this vaccine. (1/13)
https://www.jnj.com/johnson-johnson-announces-submission-of-application-to-the-u-s-fda-for-emergency-use-authorization-of-its-investigational-single-shot-janssen-covid-19-vaccine-candidate
https://www.jnj.com/johnson-johnson-announces-submission-of-application-to-the-u-s-fda-for-emergency-use-authorization-of-its-investigational-single-shot-janssen-covid-19-vaccine-candidate
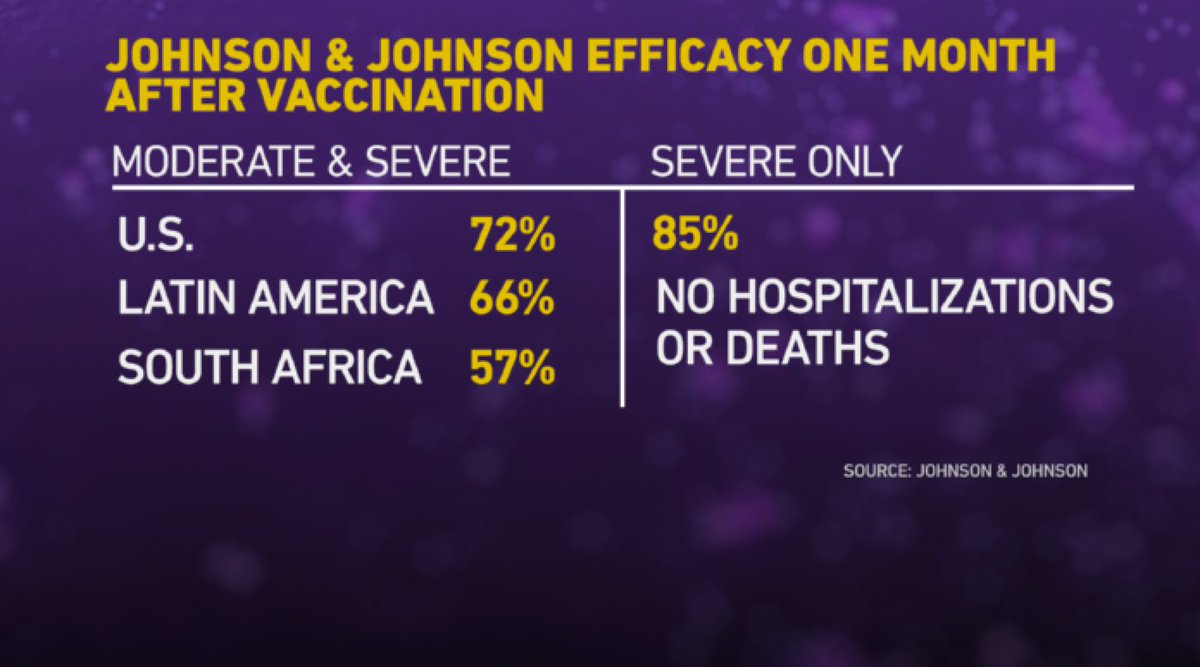
Globally, here are the results for this single-shot vaccine.
This was from one month following the shot. (2/13)
This was from one month following the shot. (2/13)
That 85% figure is important because as Dr. Fauci of @NIAIDNews said, "If you can prevent severe disease in a high percentage of individuals, that will alleviate so much of the stress and human suffering and death." (3/13)
Here's more on the breakdown of that 66% number (moderate to severe disease). So, what could be going on here? (4/13)
In South Africa, 95% of Covid-19 trial cases were caused by a variant known as B.1.351. It seems to be more contagious & carries mutations that may make the virus less susceptible to our immune response - namely, antibodies that come from prior infections or vaccines.(5/13)
We saw similar numbers from another vaccine, Novavax, whose Phase 3 trial in the UK showed 89% efficacy, compared to 60% in the company’s Phase 2b trial in South Africa. (6/13)
The good news: that 85% number, when it comes to preventing severe disease…that finding was generally consistent across all variants and age groups. Plus, there were no hospitalizations or deaths among vaccinated people one month after the shot. (7/13)
If you’re looking at these numbers versus Moderna/Pfizer, it’s hard to make a comparison at this point. For one, those mRNA vaccines showed 94-95% efficacy against symptomatic Covid-19 -- a different benchmark than “moderate to severe.” (8/13)
When I spoke with J&J’s global head of research and development, @mmammen, he also noted their trial was conducted at a time when there was more virus circulating – including variants like those first spotted in the UK and South Africa. (9/13)
So while the numbers might not look as high, this vaccine still provides significant protection and has a number of other advantages: No second dose, it can be stored at basic refrigeration temps and has a favorable side effect profile - no cases of anaphylaxis. (10/13)
Like AstraZeneca, J&J uses a weakened common cold virus that doesn’t replicate -- called adenovirus. It carries genetic instructions into the body that lead to an immune response. Pfizer and Moderna both use genetic technology called mRNA, as the basis for their vaccines (11/13)
If the FDA decides to authorize the vaccine, next the US CDC’s Advisory Committee on Immunization Practices will meet to discuss whether the vaccine should be given to Americans and if so, who should get it first. (12/14)
This same regulatory process for Pfizer took a little over three weeks. For Moderna, it was a little more than two. @mmammen believes the J&J emergency use could come by late February. (13/14)
J&J has committed 100 million doses to the US, and @mmammen says they are on track to do that by June. He told me they hope to ship doses the day after an EUA is granted. If that happens, it could have a major impact on our vaccination efforts and the vaccine rollout. (14/14)

 Read on Twitter
Read on Twitter