Our portable, in vitro conjugate vaccine expression (iVAX) platform is this week's cover @ScienceAdvances! We developed a #cellfree #synbio technology to address some of the major challenges limiting conjugate vaccine development and distribution 
 1/n https://advances.sciencemag.org/content/7/6/eabe9444
1/n https://advances.sciencemag.org/content/7/6/eabe9444

 1/n https://advances.sciencemag.org/content/7/6/eabe9444
1/n https://advances.sciencemag.org/content/7/6/eabe9444
I describe our motivation in the #preprint #tweetorial below. Main takeaway: current manufacturing platforms limit our ability to rapidly develop & distribute conjugate vaccines, which is especially concerning given the rise of drug-resistant bacteria 2/n https://twitter.com/JessicaCStark/status/1144310694361407492?s=20
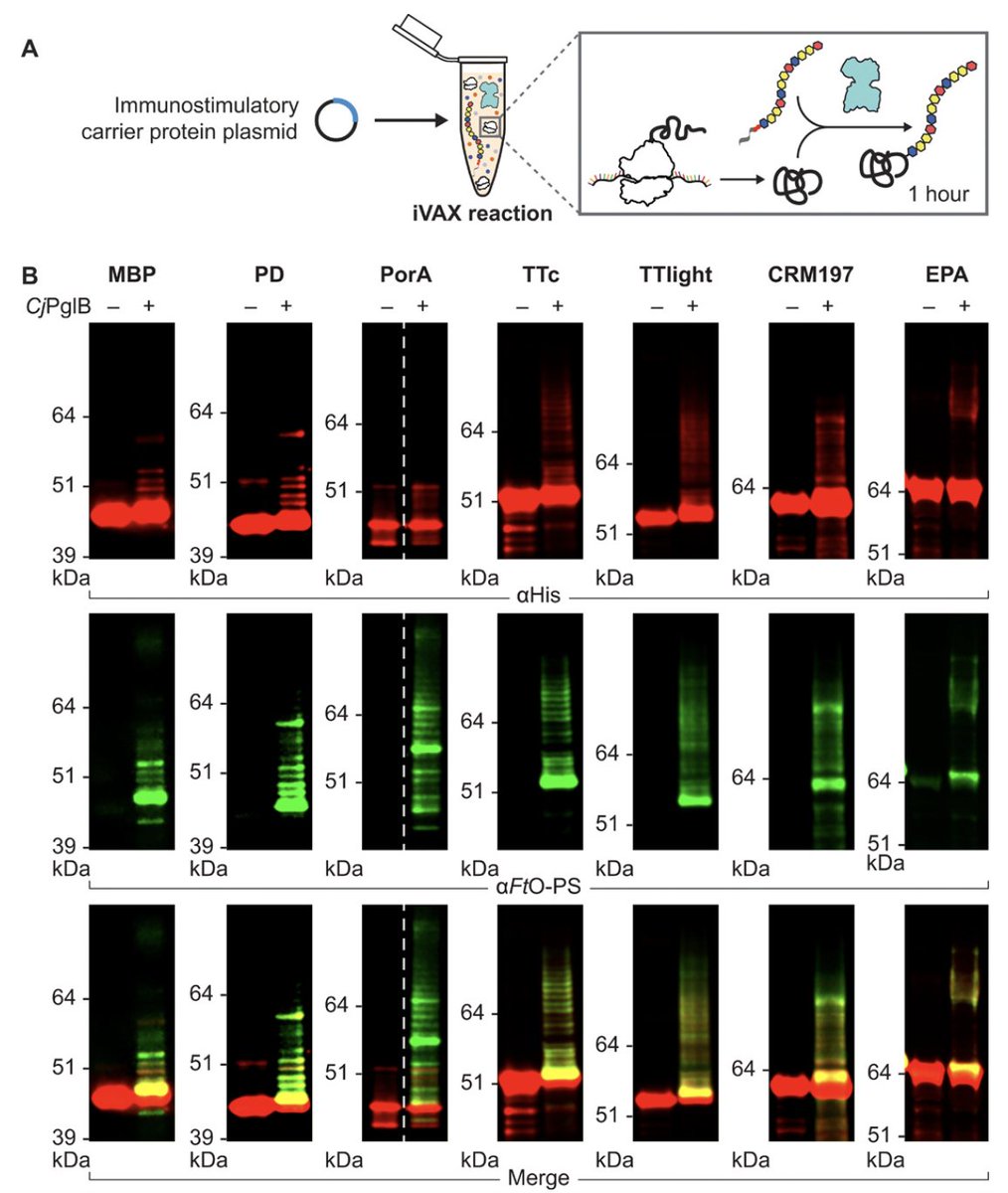
With iVAX, we demonstrated rapid (1 hr) production of conjugate vaccines targeting multiple bacterial pathogens and using an array of carrier proteins, including those found in licensed vaccines. This capability promises to facilitate novel conjugate vaccine development 3/n
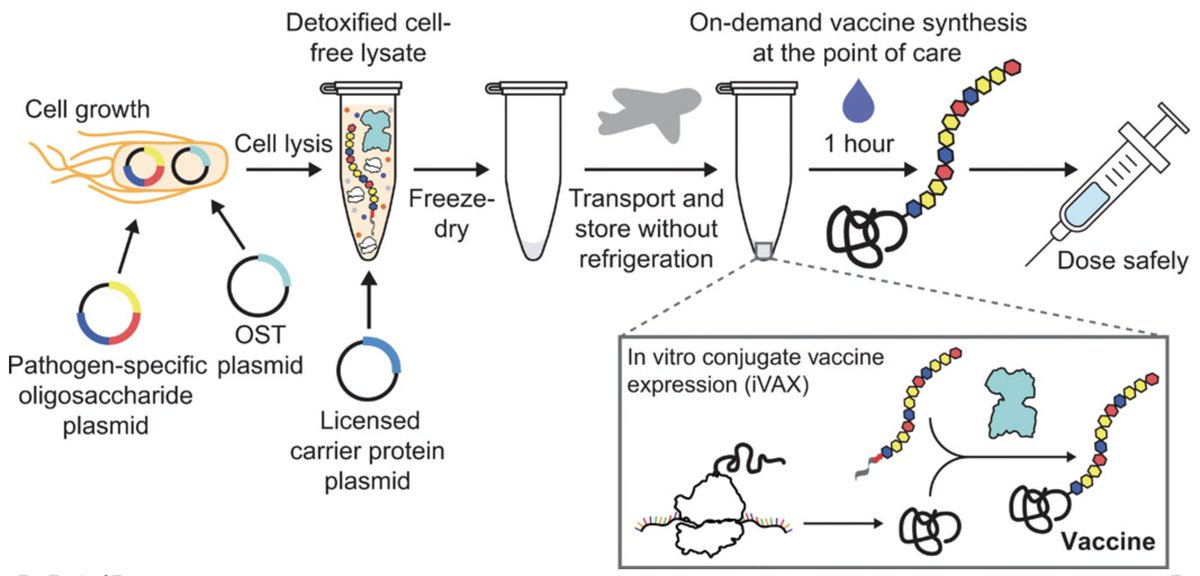
We further showed that iVAX reactions can be freeze-dried for distribution and activated on-demand by just adding water. This could reduce the need for cold chain refrigeration, which currently limits vaccination campaigns, especially in low and middle income countries 4/n
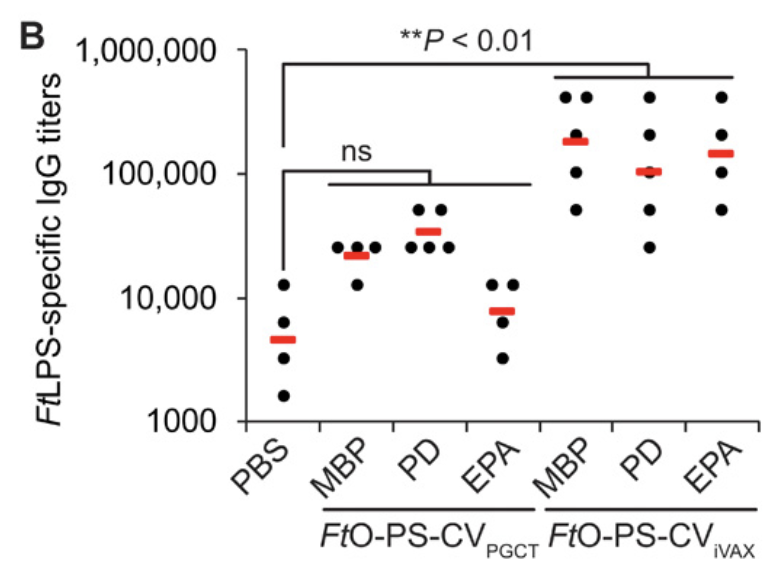
Excitingly, we showed that vaccines synthesized from freeze-dried iVAX reactions protected mice from an otherwise lethal dose of F. tularensis, a notoriously infectious and deadly bacterial pathogen that has resisted decades of vaccine development efforts 5/n
iVAX-derived vaccines thus represent the first example of an efficacious glycoprotein product made in a portable manufacturing platform. As >70% of protein therapeutics/vaccines are glycosylated, this represents an important milestone in #decentralizedmanufacturing #glycotime
One of the most surprising elements? iVAX-derived vaccines elicited higher levels of pathogen-specific, class-switched IgG antibodies than ostensibly identical vaccines made using an established technology. Further study could lead to design rules for more effective vaccines 6/n
The need to increase access to life-saving vaccines has never been more clear. We hope that iVAX can contribute to this effort through its potential for cold chain-independent distribution and point-of-care production of conjugate vaccines 7/n https://news.northwestern.edu/stories/2021/01/shelf-stable-vaccines-avoid-waste-expand-access/
A huge thank you to everyone involved, including @jewettlab_NU @Tommy_Jaroen @DeLisaGroup @jasminehershewe @ktwarfel @NUSynBio @ResearchNU @NorthwesternEng @CLP_Institute @Cornell_CBE @UIowaMicrobio

 Read on Twitter
Read on Twitter