I cannot believe the events of the last 10 days aren't getting greater attention
Events which will live on infamy as a cautionary tale of western hubris, journalistic malpractice, and the problem of media proximity to power #Covid19
THREAD
Events which will live on infamy as a cautionary tale of western hubris, journalistic malpractice, and the problem of media proximity to power #Covid19
THREAD
On January 25, German media published claims (which they didn't check or verify) of a German government source that the AstraZeneca vaccine was only 8 percent effective among over 65s
This was plain wrong. It's not clear where the 8 percent figure came from but it appears to have been confused with the proportion of over 65s included in the AZ clinical trials (i.e. -/+ 8%) https://fullfact.org/health/german-astrazeneca-8-percent-handelsblatt/
A brief aside:
8 percent over 65s is relatively small as a sample size.
The Pfizer/BioNTech #Covid19 vaccine trial had more than 40 percent of participants over 55
But it's still hundreds of people, all of whom tolerated the vaccine well, and who saw robust antibody response
8 percent over 65s is relatively small as a sample size.
The Pfizer/BioNTech #Covid19 vaccine trial had more than 40 percent of participants over 55
But it's still hundreds of people, all of whom tolerated the vaccine well, and who saw robust antibody response
Later on the 25th, AstraZeneca responds to the "completely incorrect" German media reports:
"In November, we published data demonstrating that older adults showed strong immune responses to the vaccine, with 100% of older adults generating antibodies" https://www.rfi.fr/en/astrazeneca-rejects-incorrect-reports-on-covid-19-jab-efficacy-in-elderly
"In November, we published data demonstrating that older adults showed strong immune responses to the vaccine, with 100% of older adults generating antibodies" https://www.rfi.fr/en/astrazeneca-rejects-incorrect-reports-on-covid-19-jab-efficacy-in-elderly
At this point, this non-story should have ended. The media in question was ethically (and I would argue legally) bound to issue a full correction
This whole sorry episode of non-specialists messing up their #Covid19 reporting ought to have been consigned to the scrapheap
This whole sorry episode of non-specialists messing up their #Covid19 reporting ought to have been consigned to the scrapheap
January 26: The German paper, instead of acknowledging its error, essentially doubles down while slightly changing its story.
It's apparently now a question of lack of *safety* data for the AZ vaccine among over-65s https://www.handelsblatt.com/politik/deutschland/pandemiebekaempfung-kontroverse-um-impfstoff-von-astra-zeneca/26854288.html?ticket=ST-37916-5jwVnCeZMoVht0RbeADY-ap2
It's apparently now a question of lack of *safety* data for the AZ vaccine among over-65s https://www.handelsblatt.com/politik/deutschland/pandemiebekaempfung-kontroverse-um-impfstoff-von-astra-zeneca/26854288.html?ticket=ST-37916-5jwVnCeZMoVht0RbeADY-ap2
As I mentioned, there is a *relative* paucity of data for the AstraZeneca jab among over 65s compared with Pfizer/BioNTech and Moderna vaccines
But there's not none. All expert reaction to the peer reviewed was satisfied that the AZ jab was safe/effective https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32661-1/fulltext
But there's not none. All expert reaction to the peer reviewed was satisfied that the AZ jab was safe/effective https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32661-1/fulltext
But here's the thing. On January 28, the German vaccine advisory board seems to have picked up this manufactured concern about "insufficient data" on AZ vaccine safety/efficacy among over 65s
It advises that only under 65s receive the vaccine https://www.dw.com/en/coronavirus-germany-recommends-astrazeneca-vaccine-for-under-65s-only/a-56371850
It advises that only under 65s receive the vaccine https://www.dw.com/en/coronavirus-germany-recommends-astrazeneca-vaccine-for-under-65s-only/a-56371850
Later that day, scientists, the UK prime minister and AstraZeneca itself defend the vaccine's efficacy/safety among over 65s, saying data from peer-reviewed phase 3 trials was "very reassuring" https://www.bbc.com/news/uk-55847387
January 29:
The EU's medicines agency officially recommends the AZ #Covid19 vaccine for "in people from 18 years of age"
So that's the EU's official drug agency endorsing the AZ vaccine as safe and effective for everyone, over 65s included https://news.yahoo.com/eu-regulator-backs-astrazeneca-vaccine-224558892.html
The EU's medicines agency officially recommends the AZ #Covid19 vaccine for "in people from 18 years of age"
So that's the EU's official drug agency endorsing the AZ vaccine as safe and effective for everyone, over 65s included https://news.yahoo.com/eu-regulator-backs-astrazeneca-vaccine-224558892.html
Later that day, Germany's medicines board somewhat surprisingly doubles down on its recommendation, again citing "insufficient data" https://www.thelocal.de/20210129/german-panel-keeps-advice-against-astrazeneca-jabs-for-over-65s
Even later on January 29th, no less a pre-eminent epidemiologist as France's Emmanuel Macron wades in to the debate, telling reporters that the AZ vaccine is only "quasi-effective" among over 65s https://www.france24.com/en/live-news/20210129-macron-astrazeneca-vaccine-quasi-ineffective-for-over-65s
Now, as we've seen, AstraZeneca published peer-reviewed phase 3 results back in November showing "100 percent of older adults generating spike-specific antibodies after the second dose"
I'm no mathematician, but I don't think 100% is "quasi-ineffective" https://www.rfi.fr/en/astrazeneca-rejects-incorrect-reports-on-covid-19-jab-efficacy-in-elderly
I'm no mathematician, but I don't think 100% is "quasi-ineffective" https://www.rfi.fr/en/astrazeneca-rejects-incorrect-reports-on-covid-19-jab-efficacy-in-elderly
January 30: The EU approves the use of the AstraZeneca #Covid19 vaccine for all adults, and says it expects all 400 million contracted doses as planned https://timesofindia.indiatimes.com/world/europe/eu-approves-astrazeneca-jab-as-who-warns-against-vaccine-nationalism/articleshow/80599586.cms#:~:text=EU%20approves%20AstraZeneca%20jab%20as%20WHO%20warns%20against%20'vaccine%20nationalism',-AFP%20%7C%20Updated%3A%20Jan&text=AMSTERDAM%3A%20The%20European%20Union%20on,will%20only%20prolong%20the%20pandemic.
At this point, it's worth taking a moment to look at the logistics of vaccination
Anyone who has ever had to organise mass vaccination campaigns will tell you that having the vaccine is the easy part. Getting it in to everyone's arm is the struggle
Anyone who has ever had to organise mass vaccination campaigns will tell you that having the vaccine is the easy part. Getting it in to everyone's arm is the struggle
One key barrier to successful vaccination programmes is storage
As you know, Pfizer/BioNTech and Moderna's vaccines are mRNA vaccines. These need to be stored at ultra low temperatures (-80C/-20C) or else they perish
As you know, Pfizer/BioNTech and Moderna's vaccines are mRNA vaccines. These need to be stored at ultra low temperatures (-80C/-20C) or else they perish
The AstraZeneca vaccine is a viral vector vaccine. While these require "growing" in factories they are far more robust than mRNA vaccines and don't require the same cold-chain
It can be kept for 6 months in the fridge. This is a godsend for distributors https://www.prevention.com/health/a35118263/astrazeneca-vs-pfizer-vs-moderna-covid-19-vaccine/
It can be kept for 6 months in the fridge. This is a godsend for distributors https://www.prevention.com/health/a35118263/astrazeneca-vs-pfizer-vs-moderna-covid-19-vaccine/
Then there is the issue of cost.
I know we shouldn't be able to put a price on health, but we all do, constantly.
So, costs per dose (remember you need 2), per #Covid19 vaccine:
Pfizer/BioNTech: $20
Moderna: $35
AstraZenca: $3 https://www.cnbc.com/2020/11/17/covid-vaccines-how-much-they-cost-whos-bought-them-and-how-theyre-stored.html
I know we shouldn't be able to put a price on health, but we all do, constantly.
So, costs per dose (remember you need 2), per #Covid19 vaccine:
Pfizer/BioNTech: $20
Moderna: $35
AstraZenca: $3 https://www.cnbc.com/2020/11/17/covid-vaccines-how-much-they-cost-whos-bought-them-and-how-theyre-stored.html
So this is a vaccine that is not only significantly easier to store than its competitors, it is nearly an order of magnitude cheaper
But I digress
But I digress
January 30: Italy's medicine agency recommends "alternatives" to the AstraZeneca #Covid19 vaccine be given to over 55s https://www.thelocal.it/20210131/italy-agency-cautious-on-astrazeneca-jab-for-over-55s
February 2: France's medicine agency recommends against giving the AstraZeneca vaccine to over 65s https://finance.yahoo.com/news/france-says-no-astrazeneca-virus-190751282.html
Less than 24 hrs later, a new study confirms that the AstraZeneca vaccine controls #Covid19 by two-thirds among all age groups
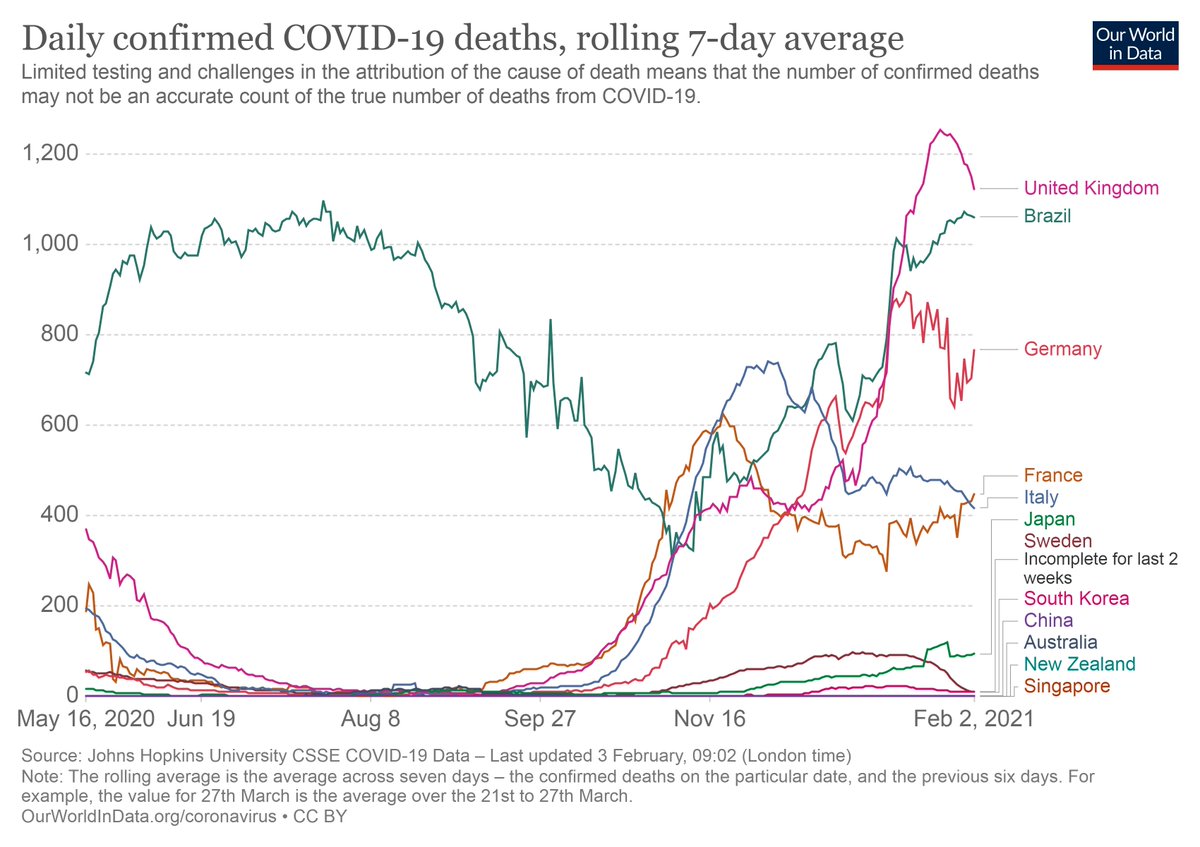
This is again a godsend for countries (like France and Germany) struggling with 100s of daily Covid deaths https://www.france24.com/en/live-news/20210203-oxford-astrazeneca-vaccine-cuts-virus-transmission-study
This is again a godsend for countries (like France and Germany) struggling with 100s of daily Covid deaths https://www.france24.com/en/live-news/20210203-oxford-astrazeneca-vaccine-cuts-virus-transmission-study
So, to sum up:
What started out as a newspaper error has been allowed to escalate to a point where two rich European nations, whose citizens are dying in their 10,000s, end up denying their most vulnerable population a safe, cheap, easily-storable and effective #Covid19 vaccine
What started out as a newspaper error has been allowed to escalate to a point where two rich European nations, whose citizens are dying in their 10,000s, end up denying their most vulnerable population a safe, cheap, easily-storable and effective #Covid19 vaccine
Epilogue:
None of this happened while we were still working out what #Covid19 is, how it transmits and kills, or what vaccines work well against it
This is a year into a pandemic in which governments vowed to move hell and high water to protect their populations
None of this happened while we were still working out what #Covid19 is, how it transmits and kills, or what vaccines work well against it
This is a year into a pandemic in which governments vowed to move hell and high water to protect their populations
And I fear that voters in Western nations have been conditioned to believe that 10,000s of #Covid19 deaths were unavoidable, as if there was no country on Earth that could have done more to prevent such horror

 Read on Twitter
Read on Twitter