A thread on our exciting discovery of niRNAs that determines plant paternal epigenetic inheritance and the comprehensive mechanism via their trans activity at both locus and cellular levels! https://www.biorxiv.org/content/10.1101/2021.01.25.428150v1
0/14
0/14
Previous work from my PhD showed that DNA methylation in the Arabidopsis male germline is reprogrammed to target and regulate genes (MetGenes), and thereby promote meiosis. https://www.nature.com/articles/s41588-017-0008-5
1/14
1/14
This methylation is catalyzed by the RNA-directed DNA methylation pathway (RdDM) that targets transposons. So how these genes are specifically targeted in the male germline was a mystery.
2/14
2/14
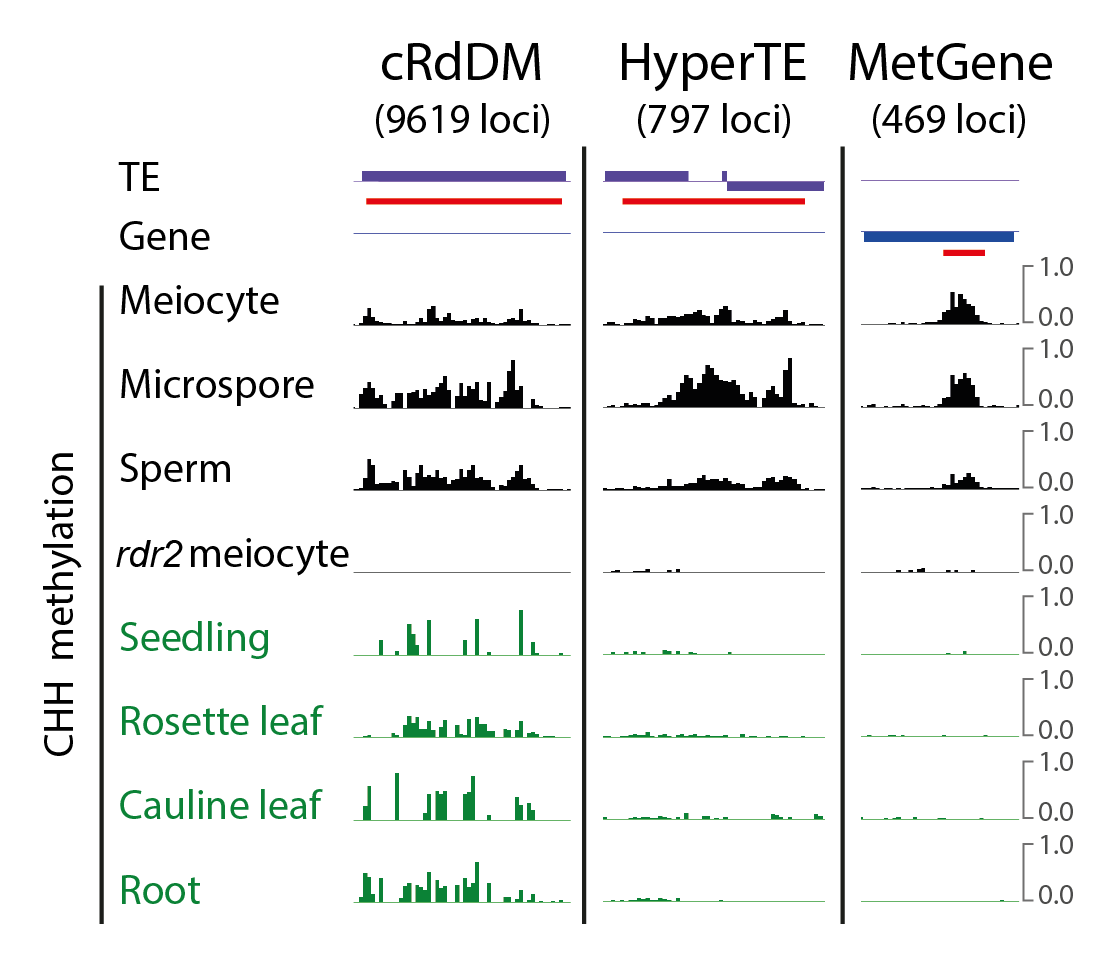
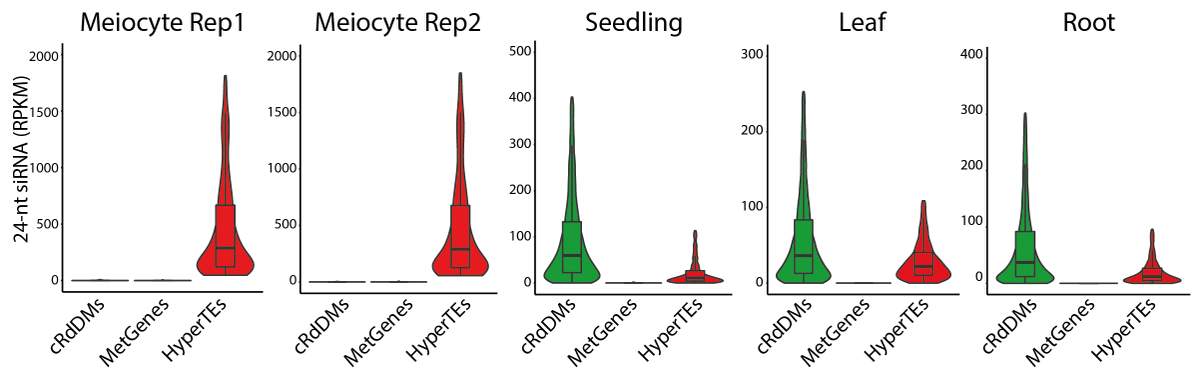
We examined sRNA from Arabidopsis meiocytes. Canonical RdDM loci have little 24-nt sRNA and methylation compared to soma. Instead, 24-nt sRNAs are concentrated in 797 TE clusters hypermethylated in the male germline. We refer to these loci as HyperTEs.
3/14
3/14
RdDM is self-reinforcing, i.e. DNA methylation always associates with 24-nt sRNAs. Surprisingly, we found few sRNAs associated with MetGenes. After a long search, we found MetGenes and HyperTEs share similar sequences.
4/14
4/14
We thus hypothesized MetGenes may be targeted by sRNAs produced from HyperTEs. Indeed, 24-nt sRNAs generated from HyperTEs can be aligned to MetGenes if mismatches are allowed.
5/14
5/14
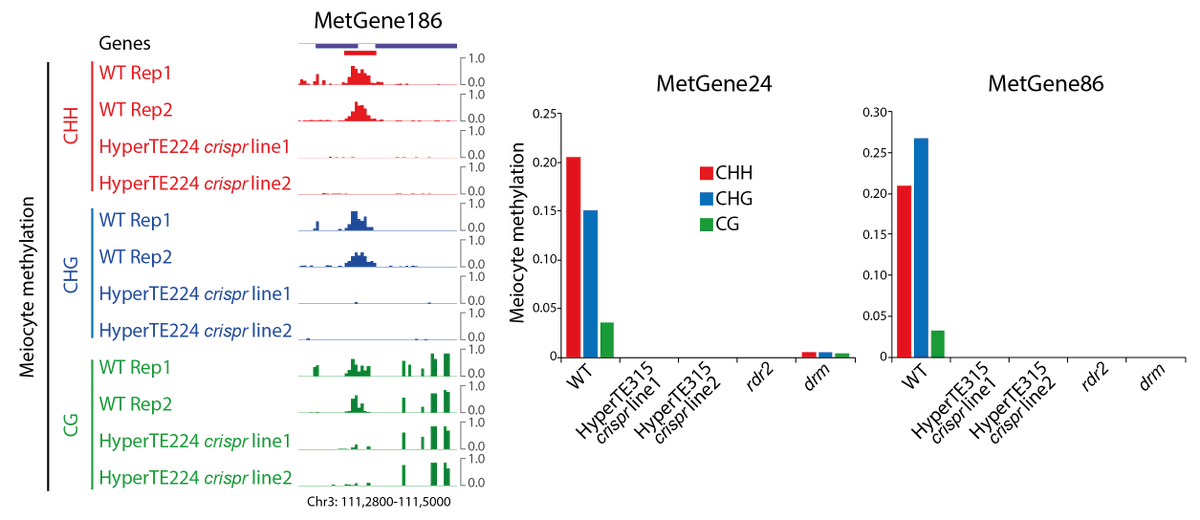
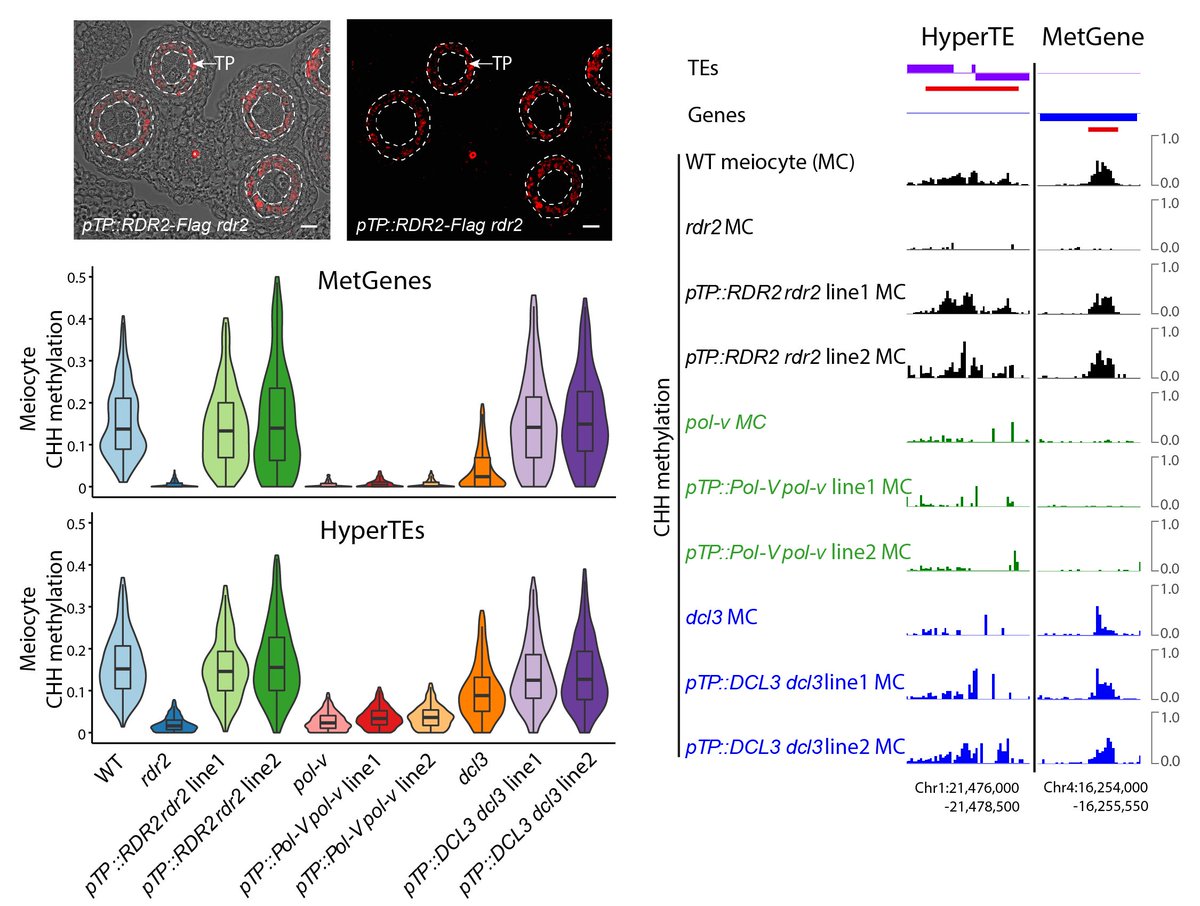
To test the causal relationship, we created CRISPR lines with deletions of two HyperTEs. We saw methylation abolishment at the target MetGenes in meiocytes. This demonstrates that methylation at MetGenes is induced in trans by sRNAs derived from HyperTEs.
6/14
6/14
Why doesn’t MetGene methylation attract Pol IV in meiocytes and produce perfect-matching sRNAs, like normal RdDM? We hypothesized that the Pol IV pathway might be suppressed in meiocytes, and the sRNAs are produced by other cells.
7/14
7/14
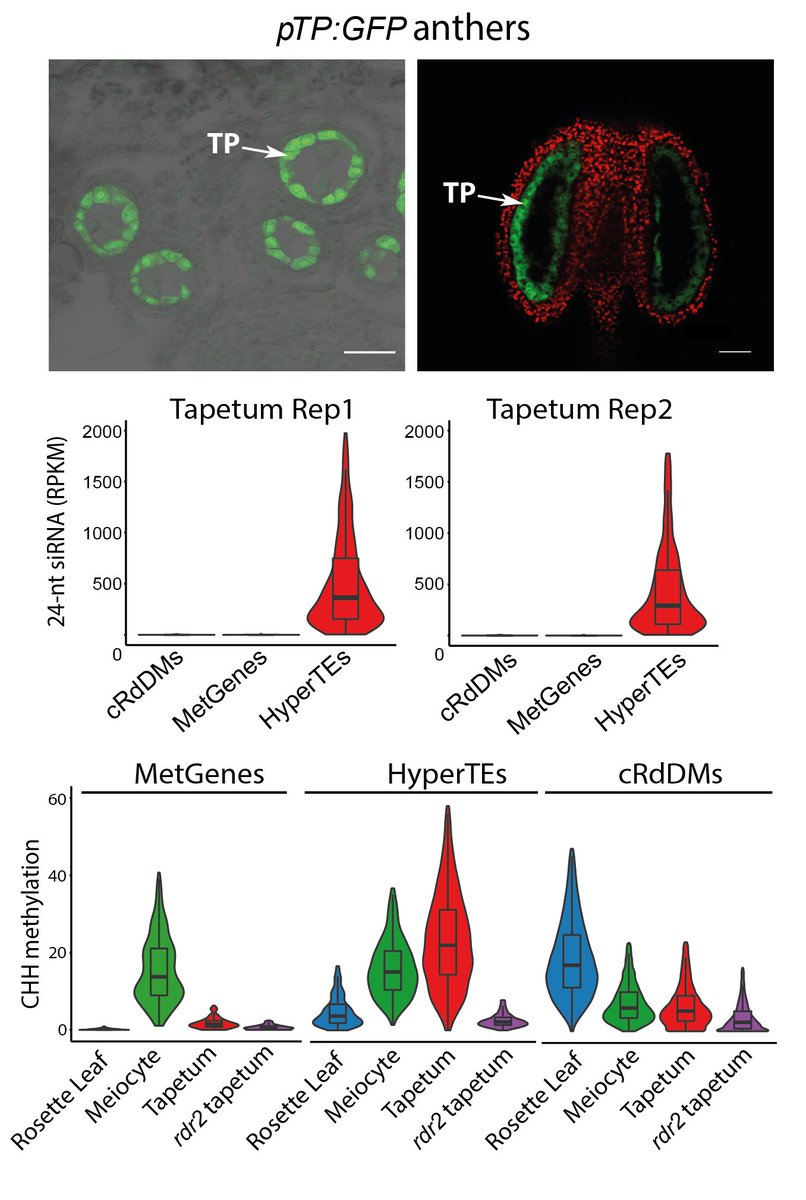
Meiocytes are enclosed by tapetal nurse cells so we developed a protocol to isolate tapetum. Tapetal and meiocyte sRNA profiles reflect each other and HyperTEs (the source of MetGene-methylating sRNAs) are hypermethylated.
8/14
8/14
Importantly, the tapetum doesn’t have MetGene methylation (hence no perfectly-matching sRNA in meiocytes).
9/14
9/14
We created several genetic mosaics with 24-nt sRNA biogenesis only in tapetum to test intercellular movement, directly demonstrating their full capability in driving meiotic methylation at MetGenes/HyperTEs. Hence, we call these nurse cell-derived sRNAs (niRNAs).
10/14
10/14
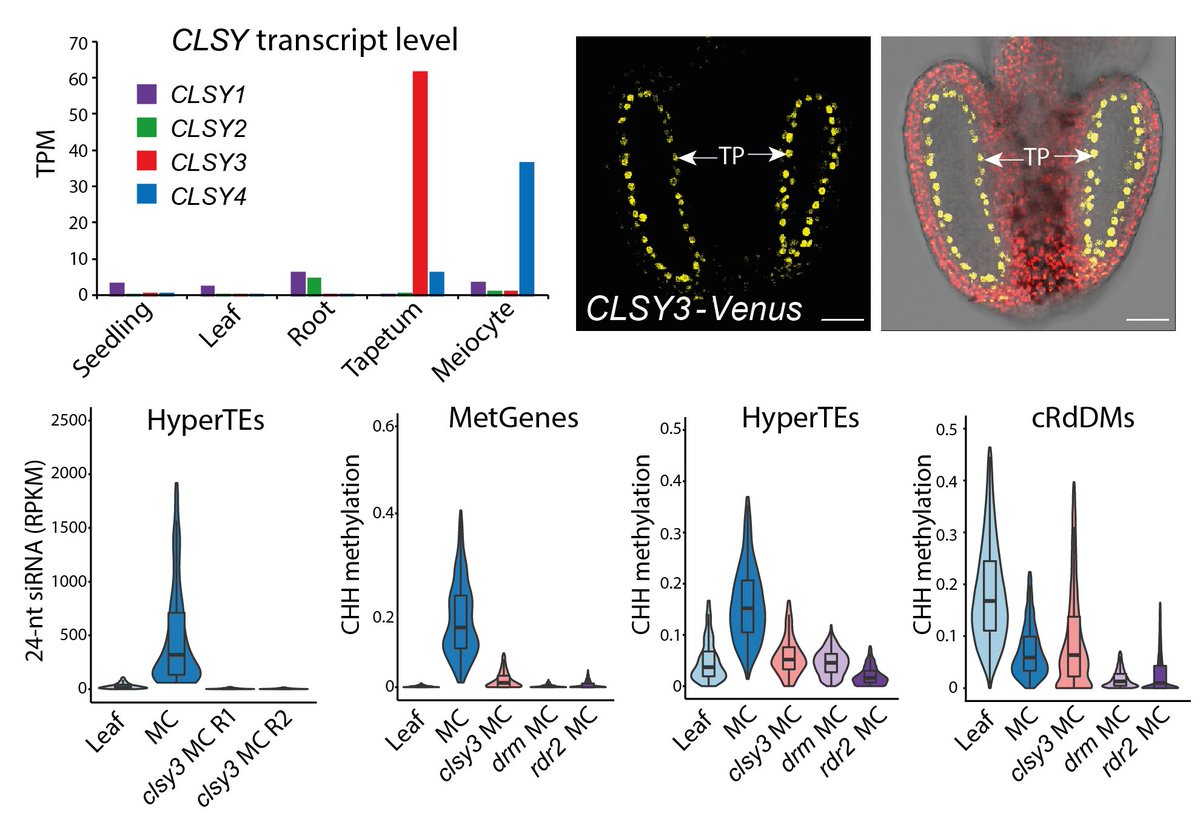
How are niRNAs produced from HyperTEs? We found strong overlap with known chromatin remodeler CLSY3-dependent sRNA clusters. CLSY3 is highly expressed in the tapetum but absent from meiocytes and soma. niRNAs and methylation are completely lost in clsy3 mutant meiocytes.
11/14
11/14
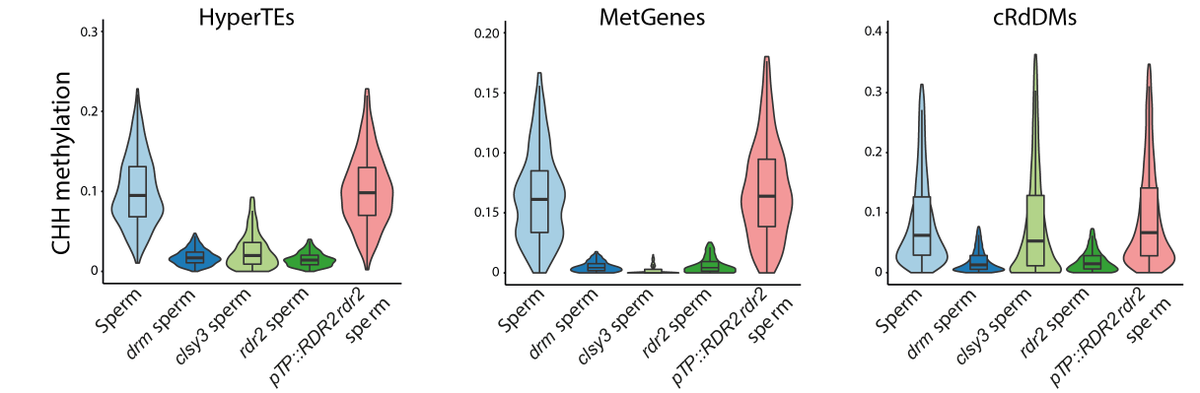
MetGene/HyperTE methylation is also reduced in clsy3 mutant sperm. Rescue of sRNA production in the tapetum restores their methylation and canonical RdDM loci. This surprising result shows tapetal niRNAs determine DNA methylation in the entire genome of the male germline.
12/14
12/14
We found Gypsy retrotransposons (GP1) are specifically activated in RdDM-defective germ cells and tapetum. With our mosaic system we show tapetal niRNAs silence GP1, a canonical RdDM locus, further validating the potency and function of tapetal niRNAs beyond HyperTEs.
13/14
13/14
Together, our paper presents six major results:
1. Meiocytes are quiescent in sRNA biogenesis; gene methylation in meiocytes is induced solely by
niRNAs transported from tapetal nurse cells.
2. Gene-targeting niRNAs are produced by TEs with imperfectly matching sequences.
1. Meiocytes are quiescent in sRNA biogenesis; gene methylation in meiocytes is induced solely by
niRNAs transported from tapetal nurse cells.
2. Gene-targeting niRNAs are produced by TEs with imperfectly matching sequences.
3. Meiocytes are uniquely able to use imperfectly matching sRNAs to target DNA methylation.
4. A remodeler specific to tapetal cells, CLSY3, drives the biogenesis of gene-targeting niRNAs.
4. A remodeler specific to tapetal cells, CLSY3, drives the biogenesis of gene-targeting niRNAs.
5. Tapetal niRNAs can specify the entire methylome of the male germline, including the sperm.
6. niRNAs silence TEs in the germline and protect genome integrity. (14/14)
6. niRNAs silence TEs in the germline and protect genome integrity. (14/14)
This was such a cool project for me to work on to uncover some important mysteries about RdDM and sRNAs. Huge thanks and congratulations to all those involved @LongJincheng, Wenjing She, @Billy_Aldridge, @Hong_bo_Gao, Sam Deans, @feng_lab, and of course @XiaoqiFeng_m
We hope you find this preprint of interest and would love to hear your questions and opinions. Thank you!

 Read on Twitter
Read on Twitter