How is a vaccine approved for production
 Pre-approval stage following clinical trials
Pre-approval stage following clinical trials
 Regulatory assessment & approval
Regulatory assessment & approval
 WHO prequalification & approval
WHO prequalification & approval
 Ready for production
Ready for production
Find out more in detail http://bit.ly/3675hhZ
http://bit.ly/3675hhZ

 Pre-approval stage following clinical trials
Pre-approval stage following clinical trials Regulatory assessment & approval
Regulatory assessment & approval WHO prequalification & approval
WHO prequalification & approval Ready for production
Ready for productionFind out more in detail
 http://bit.ly/3675hhZ
http://bit.ly/3675hhZ
Making vaccines available can take a long time, even when there’s a health emergency like #COVID19.
WHO has developed the Emergency Use Listing (EUL) to ensure rapid access for all. This video explains how
WHO has developed the Emergency Use Listing (EUL) to ensure rapid access for all. This video explains how

How is a vaccine made
Typically, companies will work to complete clinical development plans for a vaccine. Once authorized, manufacturing begins to scale up.
#COVAX will also boost development capacity & ensure equitable distribution of vaccines. http://bit.ly/3675hhZ
http://bit.ly/3675hhZ

Typically, companies will work to complete clinical development plans for a vaccine. Once authorized, manufacturing begins to scale up.
#COVAX will also boost development capacity & ensure equitable distribution of vaccines.
 http://bit.ly/3675hhZ
http://bit.ly/3675hhZ
How is a vaccine stored
Vaccines need to be stored at the correct temperature.
Most vaccines require refrigerated storage at between 2 and 8 °C. Some newer vaccines need to be kept cold at -20°C or ultra-cold at -70°C.
More info

 http://bit.ly/3675hhZ
http://bit.ly/3675hhZ

Vaccines need to be stored at the correct temperature.
Most vaccines require refrigerated storage at between 2 and 8 °C. Some newer vaccines need to be kept cold at -20°C or ultra-cold at -70°C.
More info


 http://bit.ly/3675hhZ
http://bit.ly/3675hhZ
How is a vaccine shipped
 To maintain this cold chain, vaccines are shipped using specialized equipment
To maintain this cold chain, vaccines are shipped using specialized equipment
 Refrigerated lorries transport the vaccines to the cold room
Refrigerated lorries transport the vaccines to the cold room
 Portable iceboxes are used to transport vaccines to regional centres
Portable iceboxes are used to transport vaccines to regional centres
More info http://bit.ly/3675hhZ
http://bit.ly/3675hhZ

 To maintain this cold chain, vaccines are shipped using specialized equipment
To maintain this cold chain, vaccines are shipped using specialized equipment Refrigerated lorries transport the vaccines to the cold room
Refrigerated lorries transport the vaccines to the cold room Portable iceboxes are used to transport vaccines to regional centres
Portable iceboxes are used to transport vaccines to regional centresMore info
 http://bit.ly/3675hhZ
http://bit.ly/3675hhZ
What about quality control
The safety of the vaccine is paramount. National authorities & WHO constantly monitor & conduct regular assessments & post-approval clinical studies to report on its safety & effectiveness.
More info http://bit.ly/3675hhZ https://twitter.com/WHO/status/1355426621759643649?s=20
http://bit.ly/3675hhZ https://twitter.com/WHO/status/1355426621759643649?s=20

The safety of the vaccine is paramount. National authorities & WHO constantly monitor & conduct regular assessments & post-approval clinical studies to report on its safety & effectiveness.
More info
 http://bit.ly/3675hhZ https://twitter.com/WHO/status/1355426621759643649?s=20
http://bit.ly/3675hhZ https://twitter.com/WHO/status/1355426621759643649?s=20
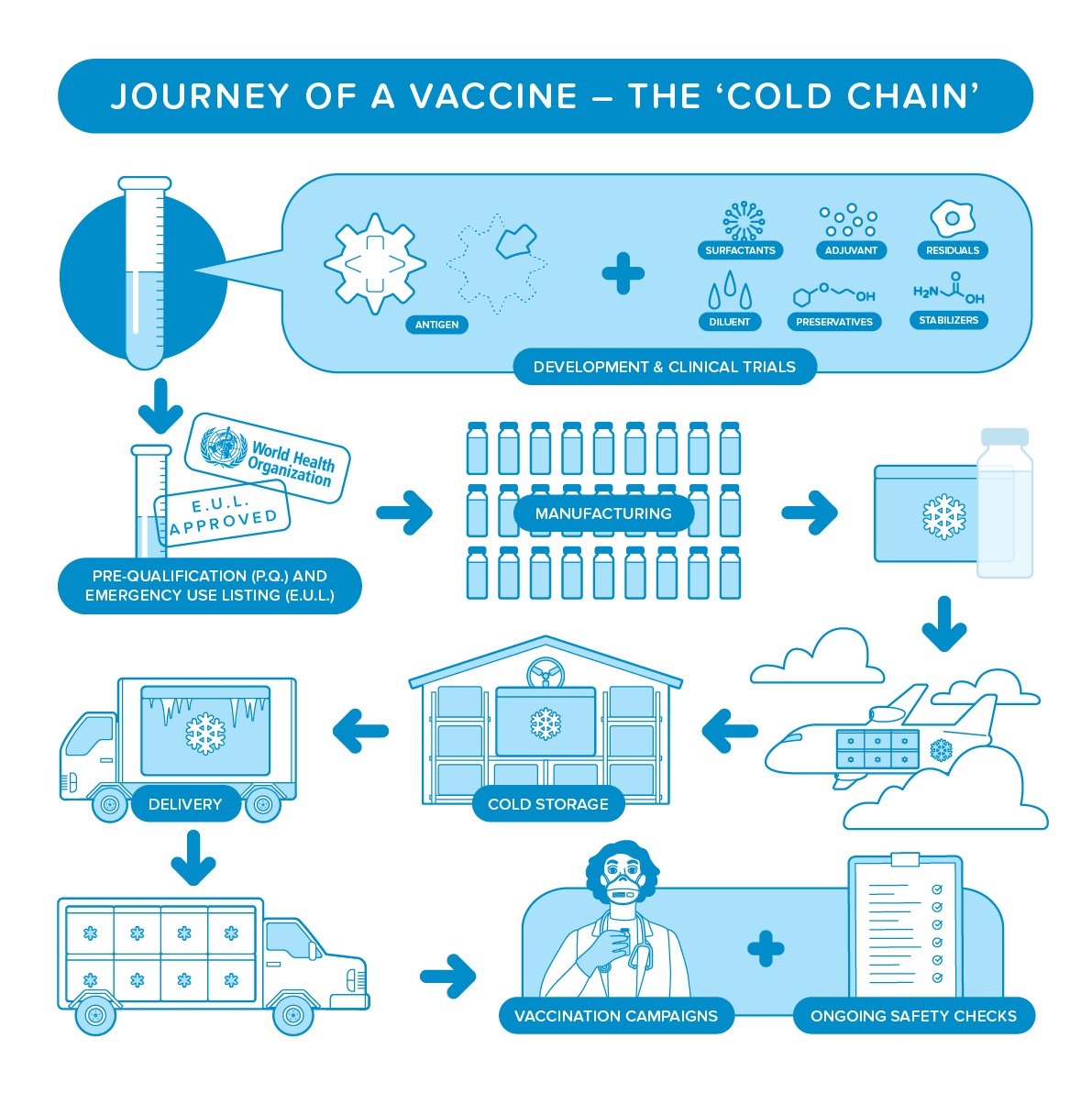
 Watch and follow the journey of a vaccine from development & clinical trials
Watch and follow the journey of a vaccine from development & clinical trials  to manufacturing & cold storage
to manufacturing & cold storage  to vaccination
to vaccination  http://bit.ly/3675hhZ ⠀
http://bit.ly/3675hhZ ⠀
Developing a vaccine is a long road full of checkpoints  to make sure it is both safe and effective.
to make sure it is both safe and effective.
 Join us on the road to #COVID19 vaccines!
Join us on the road to #COVID19 vaccines!  http://bit.ly/3675hhZ
http://bit.ly/3675hhZ
 to make sure it is both safe and effective.
to make sure it is both safe and effective. Join us on the road to #COVID19 vaccines!
Join us on the road to #COVID19 vaccines!  http://bit.ly/3675hhZ
http://bit.ly/3675hhZ

 Read on Twitter
Read on Twitter