Fresh #herpesvirus content:
Fresh #herpesvirus content:1/ We posted an updated preprint of our #singlecell functional genomics study of human #cytomegalovirus infection.
I didn't do a tweetorial with the initial version, so here it comes now

https://www.biorxiv.org/content/10.1101/775080v2
#lovevirology #herpes4life
2/ Our guiding questions were:
- What host factors and viral genes affect the progression of lytic infection?
- What actually happens when we target those critical factors? Can we prevent infection? If so, "what goes wrong?" (from the perspective of the virus).
- What host factors and viral genes affect the progression of lytic infection?
- What actually happens when we target those critical factors? Can we prevent infection? If so, "what goes wrong?" (from the perspective of the virus).
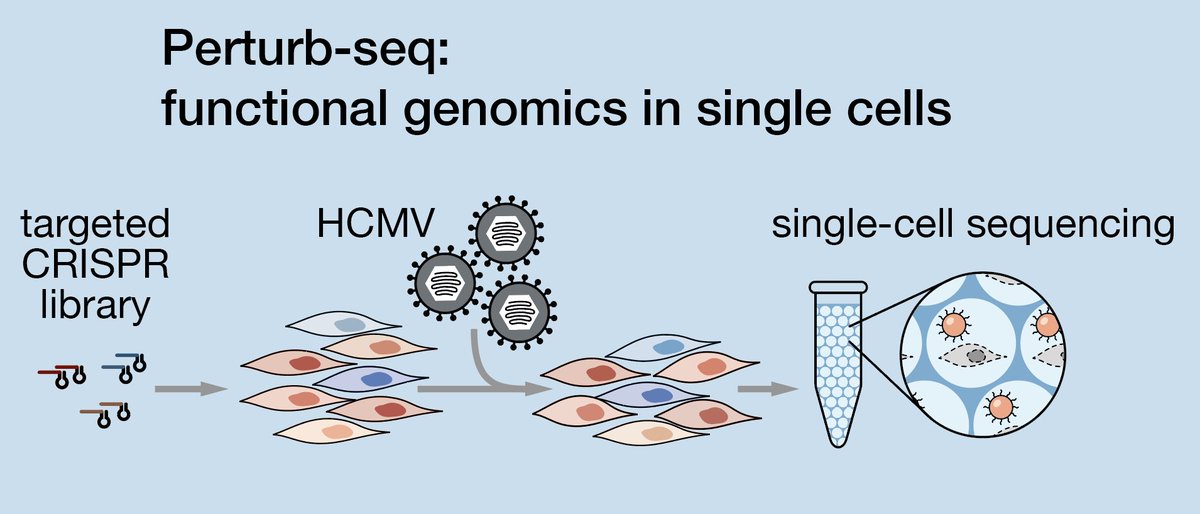
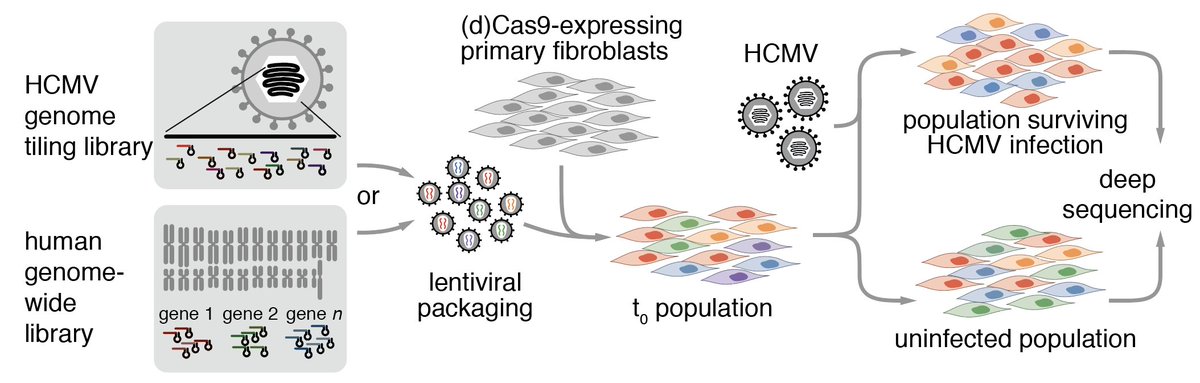
3/ We started with a series of CRISPR screens. HCMV infection kills fibroblasts over the course of a few days, but CRISPRi (knockdown) of host genes or Cas9-mediated cuts at specific positions along the viral genome can protect cells from dying, or even accelerate cell death.
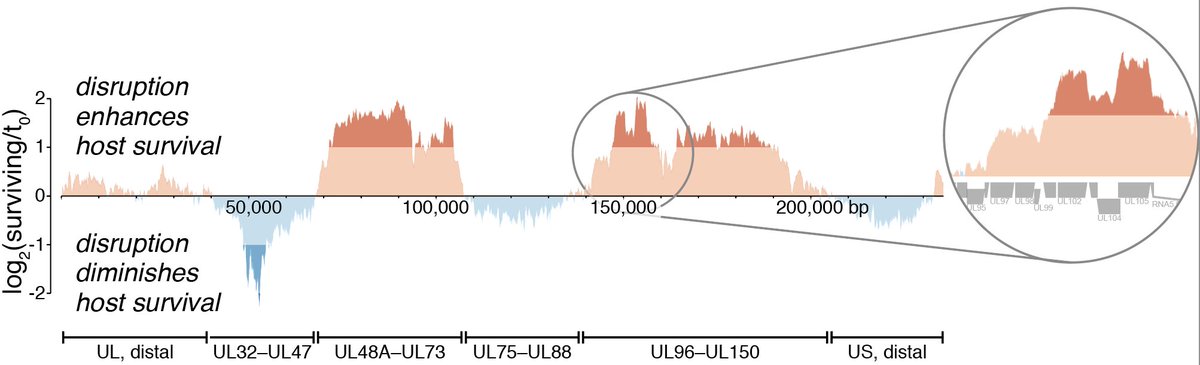
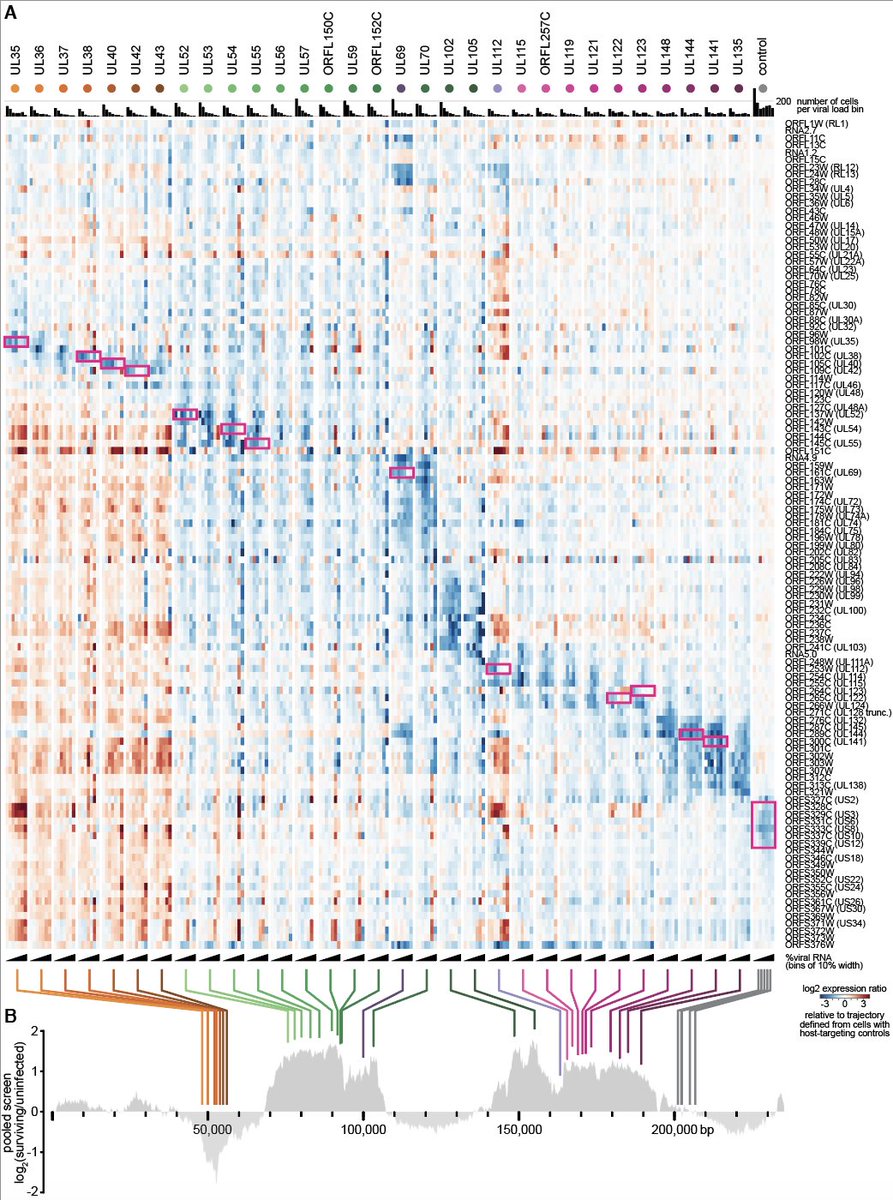
4/ The HCMV genome is very large (for a virus) and encodes hundreds of genes. It's dsDNA, so we used Cas9 and a guide library that tiles the entire genome, giving us a high-res phenotypic map. We found modules of genes with similar phenotypes, and 'cliffs' at gene
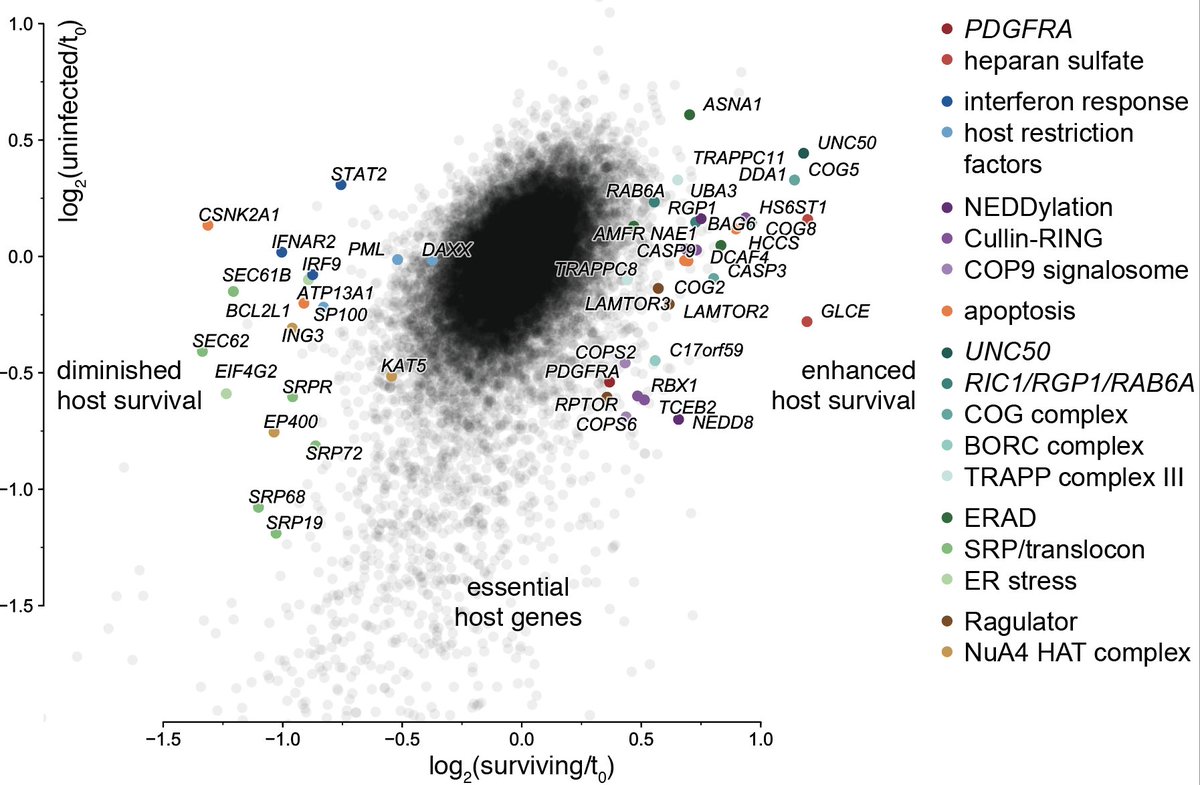
5/ The host factor screen was more of a conventional CRISPRi screen and gave us a list of candidate dependency and restriction factors from a number of pathways. But as screens do, they don't tell you why exactly a gene is a hit.
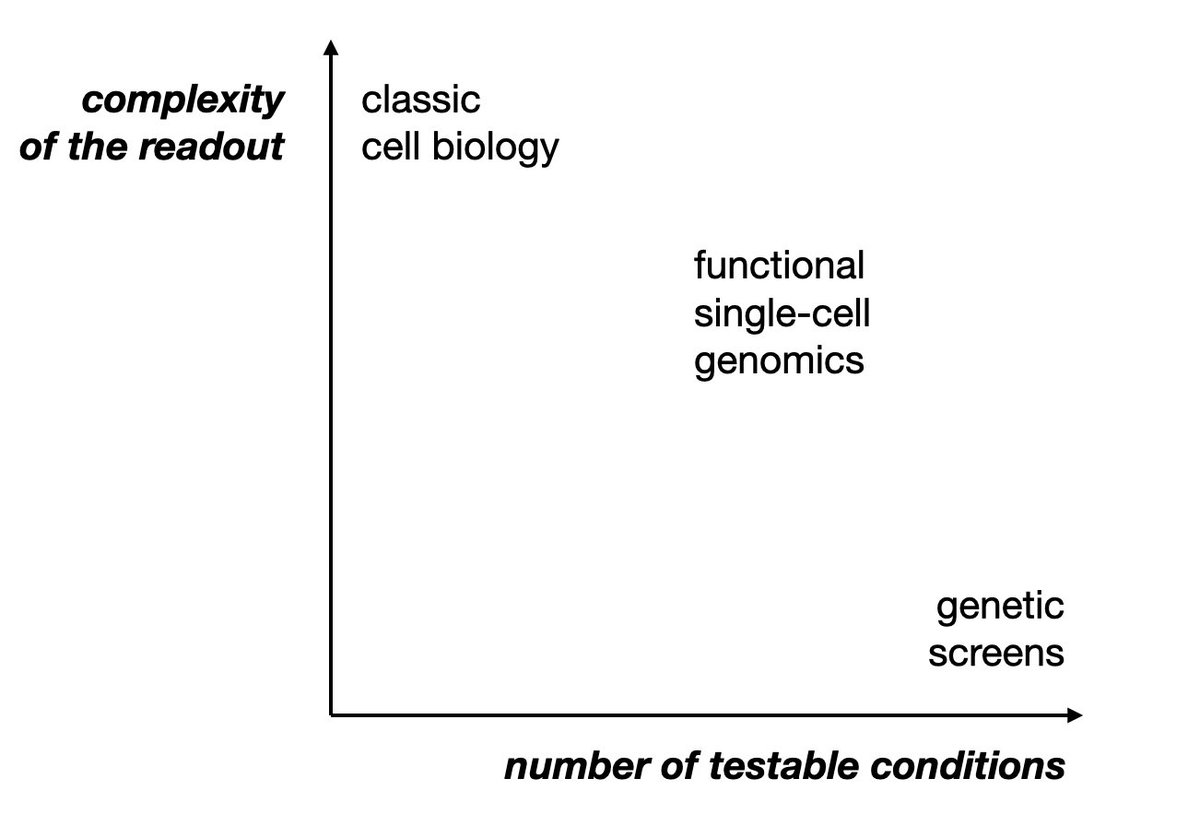
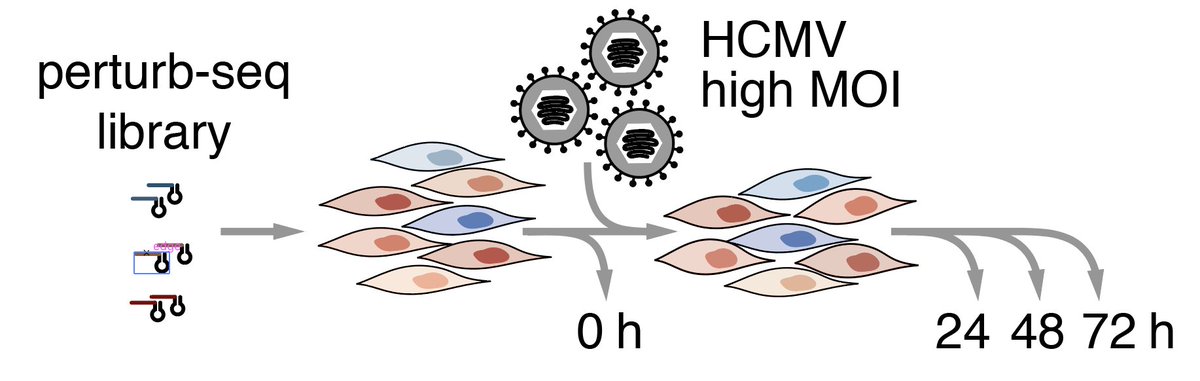
6/ This is where Perturb-seq comes in: it's functional genomics with a single-cell readout. Instead of cherry-picking a few hits for in-depth follow-up, you can characterize 'all'. Medium/high throughput, deep phenotypic readout, highly multiplexed b/c 1 cell = 1 data point.
7/ Viral infection is already highly heterogeneous at the single-cell level, as many others have shown ( @fabiousername @abrussel86 @jbloom_lab @NirDrayman @ewyler @vedranfranke @DolkenL @AE_Saliba), and with Perturb-seq, we introduce additional heterogeneity on top.
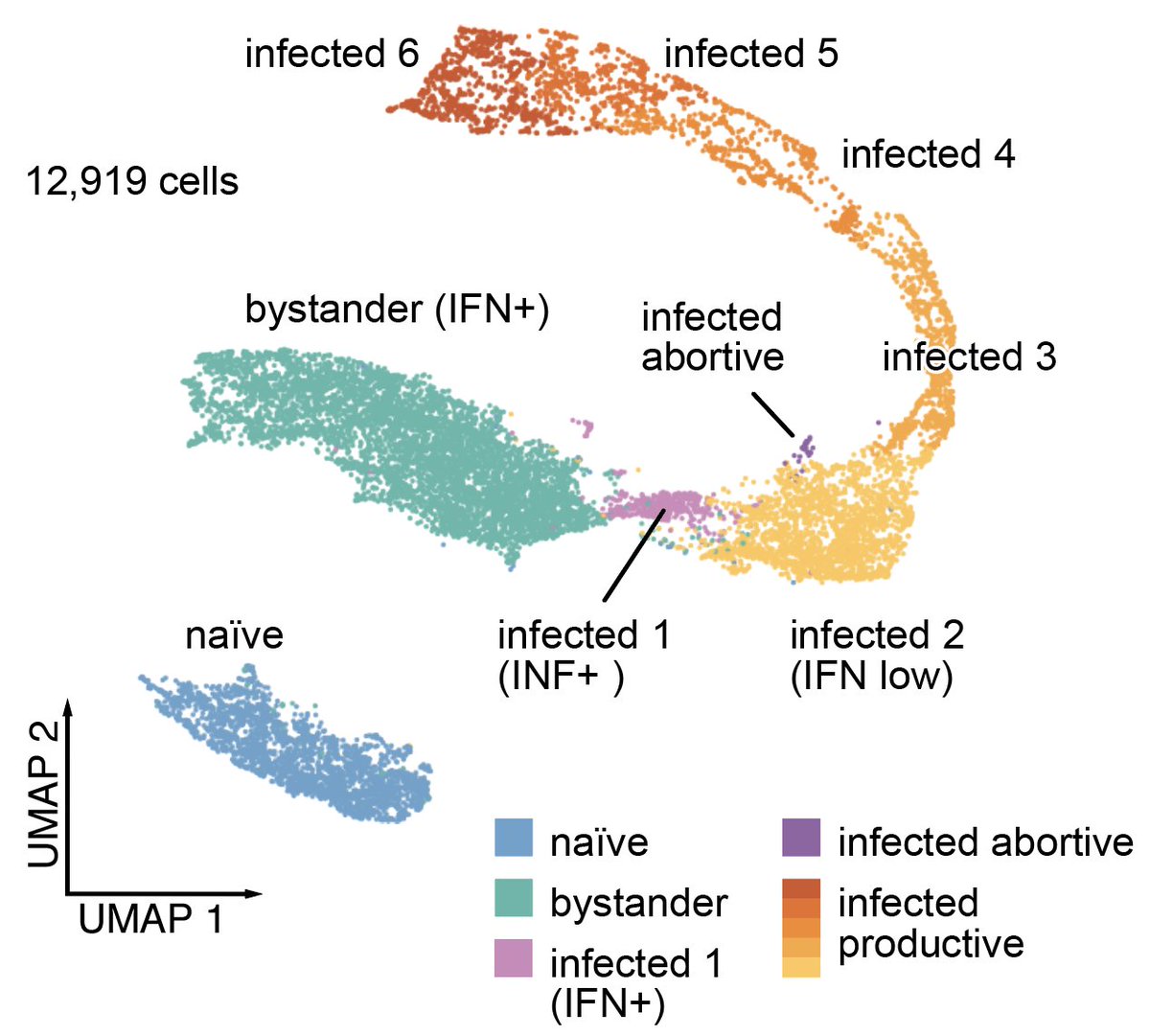
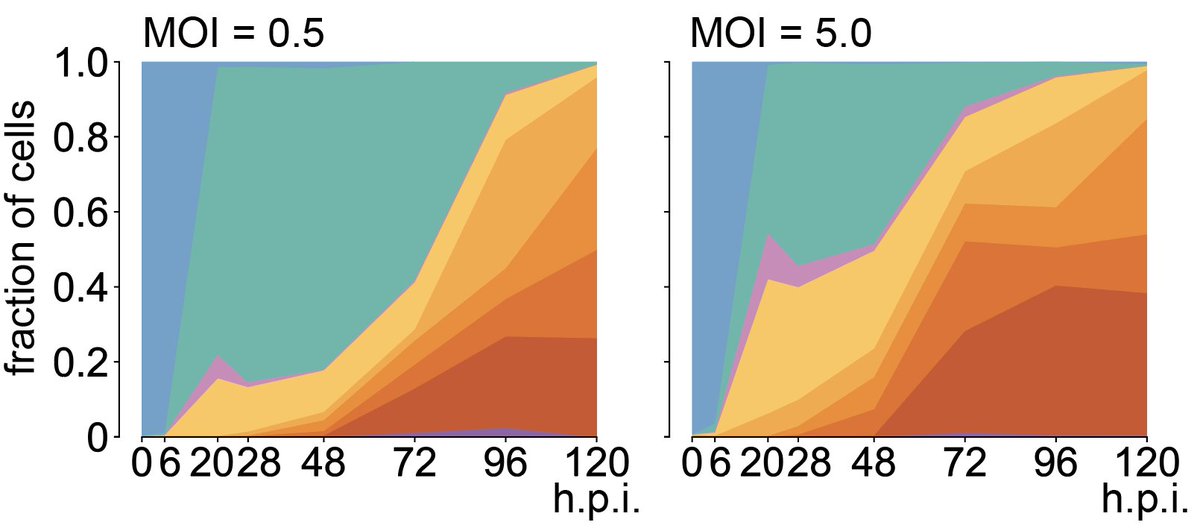
8/ First we needed a baseline for how HCMV infection progresses at the single-cell level. I'll do a separate thread on this. The gist is: fibroblasts can be either naïve, in a bystander state (uninfected, strong IFN resp), or infected (expressing viral genes, suppressed IFN resp)
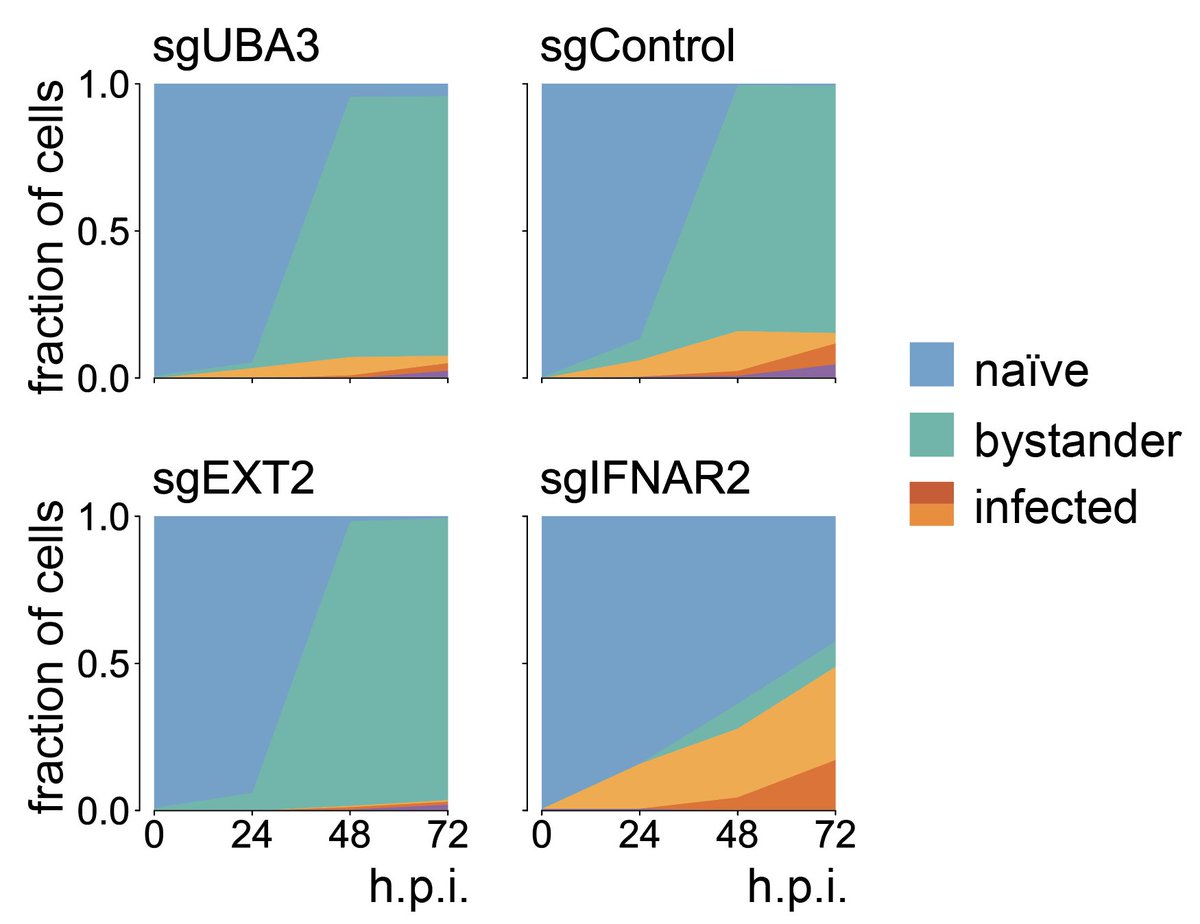
9/ How does targeting viral host factors change progression? Very much! Preventing viral adhesion (EXT2) blocks infection almost entirely. Targeting UBA3 delays progression. Targeting the IFN receptor prevents the bystander response and accelerates progression in infected cells.
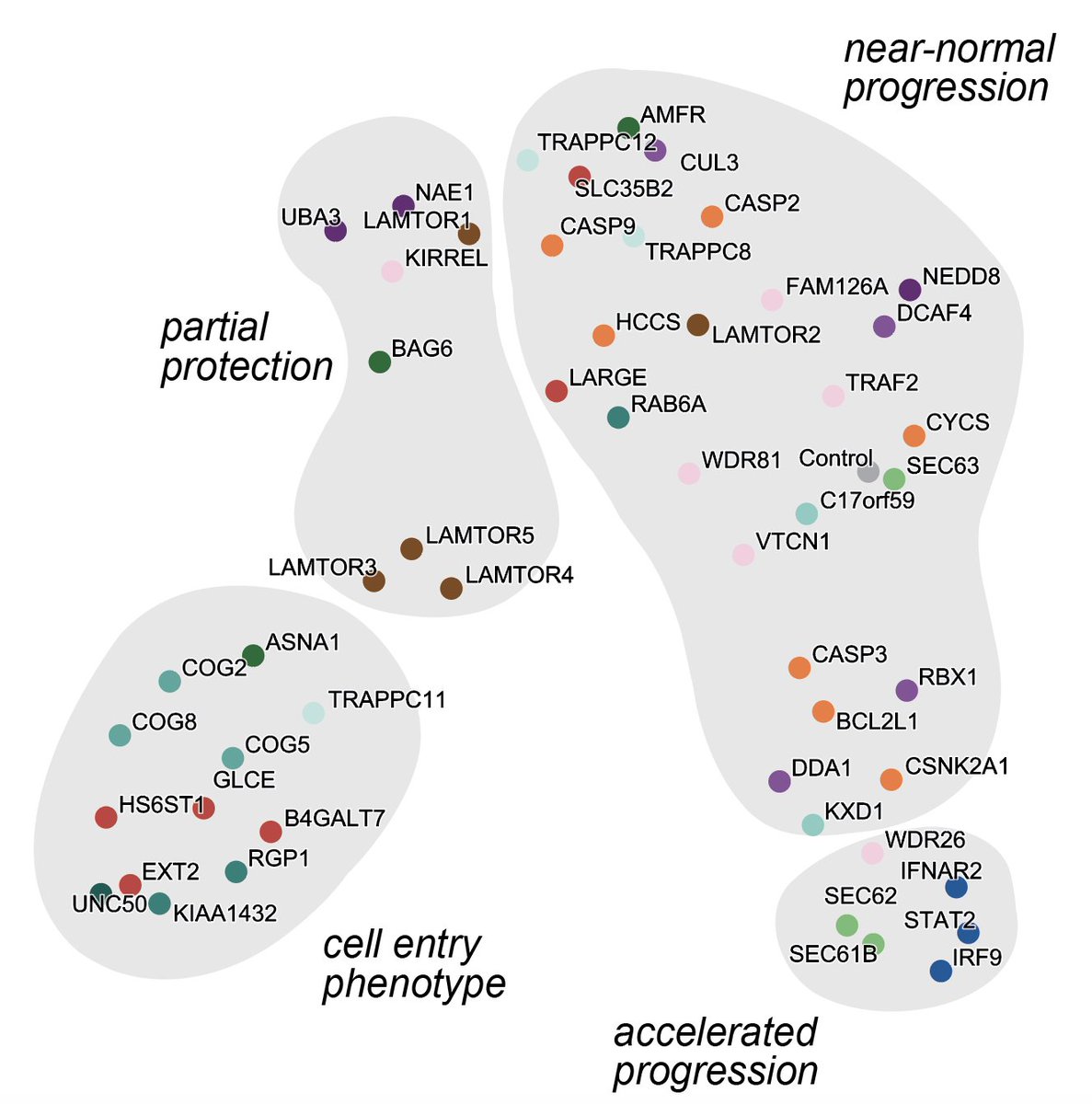
10/ now it gets a little nerdy: You can do a UMAP projection of all those cluster progression diagrams and voilà: you get a systematic classification of host factors by how they influence the progression of infection (if you annotate the plot a little bit).
11/ But what about viral factors? Great question!
We next did a CRISPRn experiment where we target both host and viral factors, and hit the cells with a lot of HCMV in order to get as many infected cells as possible.
We next did a CRISPRn experiment where we target both host and viral factors, and hit the cells with a lot of HCMV in order to get as many infected cells as possible.
12/ The most striking finding was that targeting viral genes not only changes how fast and how far cells progress during infection, but it changes the trajectory of infection, i.e. which viral genes get expressed at what time and in which quantities.
13/ One can get into a lot of detail on how exactly viral gene expression gets derailed, but the effects
- are specific to which viral gene is targeted
- seem to be an effect of the loss of the target gene, not an effect 'in cis' of the Cas9 cut.
- are again arranged in modules
- are specific to which viral gene is targeted
- seem to be an effect of the loss of the target gene, not an effect 'in cis' of the Cas9 cut.
- are again arranged in modules
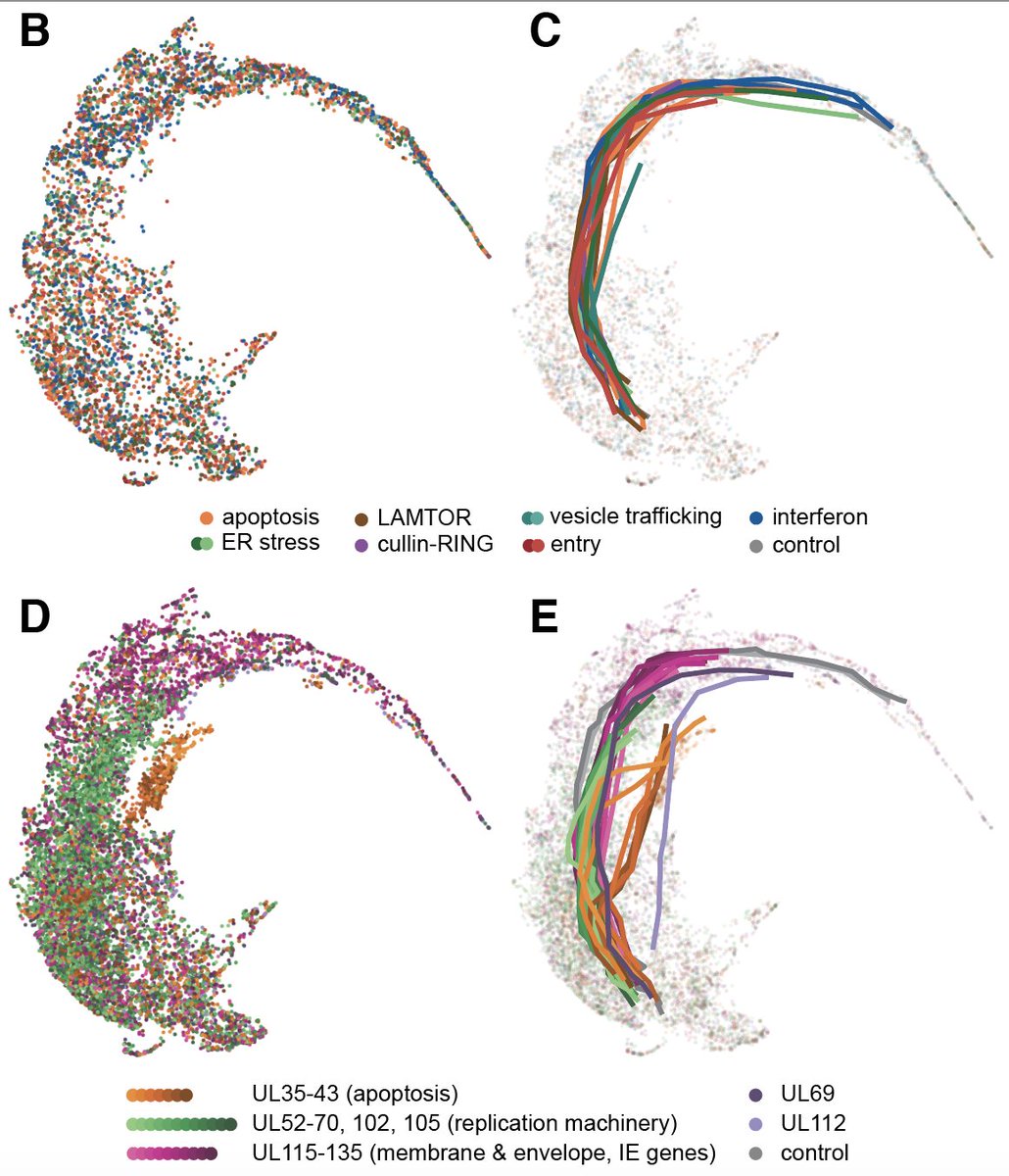
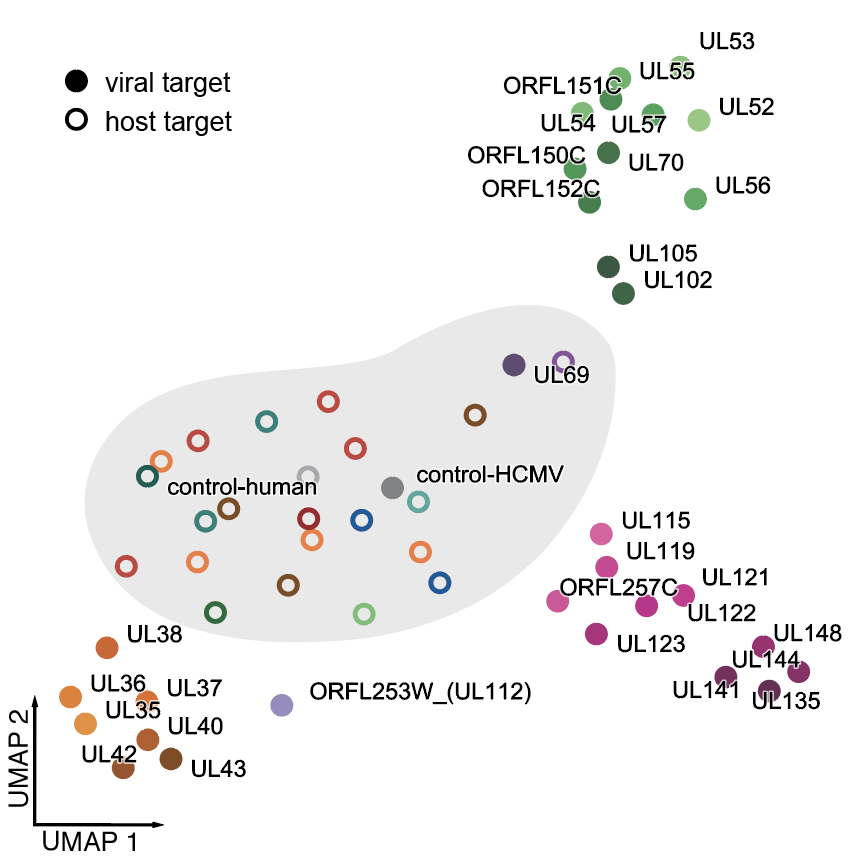
14/ and once again, UMAP is your friend: here's a picture of the similarities of the different trajectories, showing 3 main groups, plus the maverick genes UL69 and UL112. Targeting these causes distinct gene expression programs, hinting at roles in transcriptional regulation.

 Read on Twitter
Read on Twitter