I’m excited to announce that our paper showing how tumor draining lymph nodes contain a long-term, stable reservoir of anti-tumor CD8 T cells is now online at @biorxiv. This is the beautiful work of @KelliAConnolly, a postdoc in the lab. Tweetorial: https://twitter.com/biorxivpreprint/status/1354614154397687810
Tweetorial: There has been a lot of beautiful work showing that TCF1HI stem-like CD8 T Cells (TSLs) are important for sustaining T cell populations during chronic challenges and for responding to tumors, but TSLs are driven to differentiate into exhausted T cells (TEXs) 2/
by chronic antigen, cytokines. These signals are high in tumors, which raised our question: how TSLs are maintained in tumors during months-years before tumors are diagnosed. To address this, we used an autochthonous mouse lung adenocarcinoma model where developing tumors 3/
express neoantigens and we tracked tumor specific CD8 T cells over 5 months of tumor development. Surprisingly, tumors retained TCF1+ CD8s despite an overall shift towards a more differentiated T cell population and a shift in the immune microenvironment from hot to cold 4/
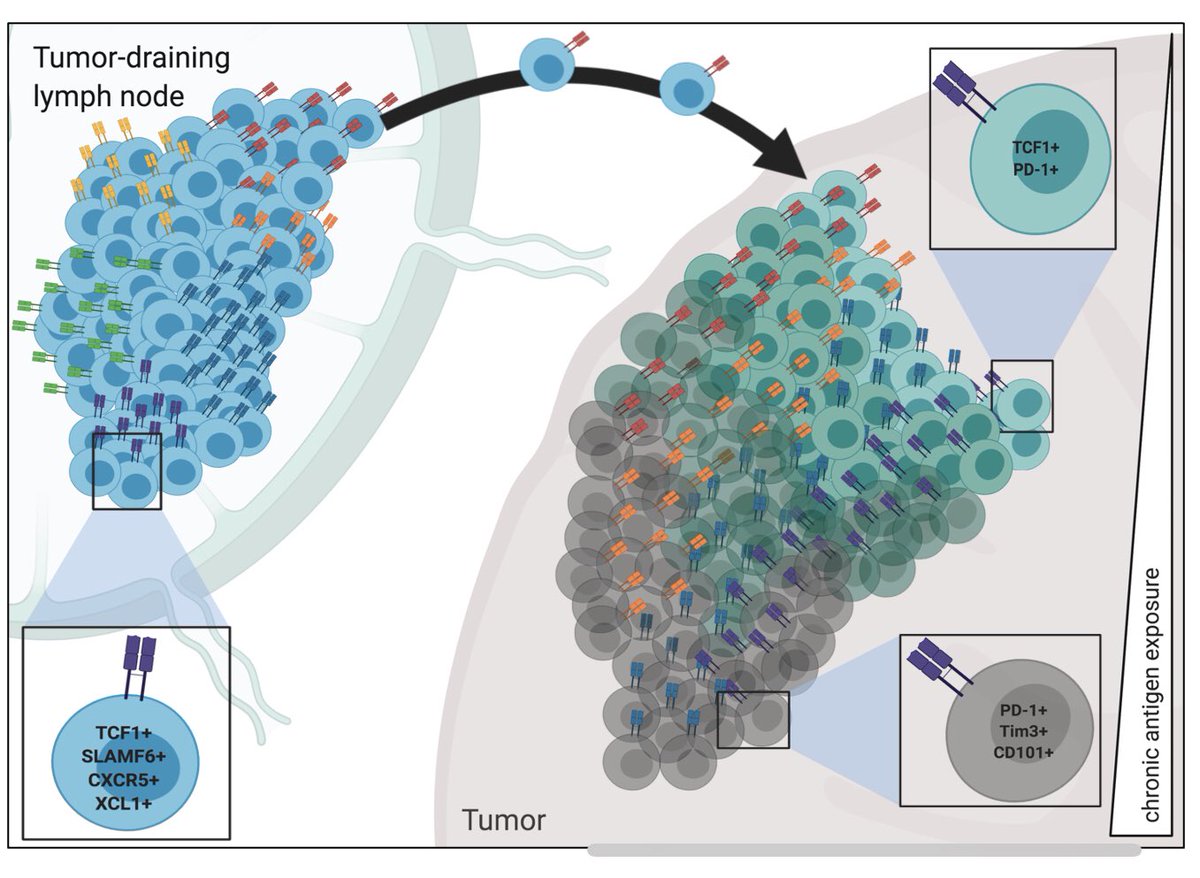
More surprisingly, tumor draining LNs (dLNs) maintained large numbers of tumor-specific CD8 T cells and nearly all had TSL phenotype (TCF1+ Slamf6+). With @KrishnaswamyLab, we analyzed single cell RNAseq/TCRseq on sorted endog tumor-specific cd8s from tumors and LNs. 5/
This shows that T cells in the tumor microenvironment were more skewed to TEX cells in late, immunologically cold tumors. By contrast, TSLs in the dLN were transcriptionally similar between 2 and 5 month tumors, highlighting the long-term stability of this population. 6/
Moreover: tumor dLNs from hot and cold tumors had similar populations of stem-like tumor-specific CD8 T cells. Further analysis showed a continuum of T cell differentiation that begins with TSLs in the dLN and ends with TEXs in the tumor, with differentiation 7/
occurring primarily in the tumor. Using TCRseq/TCR motif analysis (with Weiguo Cui), we tested the hypothesis that tumor-specific CD8 T cell clones were maintained between tumor and LN and between early and late tumors. This provided strong evidence to support the idea that 8/
tumor-specific CD8 T cell clones are maintained in the dLN over the course of tumor development. Using FTY720, we also demonstrated that tumor-specific T cells must migrate from the dLN to the tumor to maintain the TCF1HI T cells in the tumor. Finally, with Hongyu Zhao, we 9/
found evidence for TSL-like CD8 T cells in single cell RNAseq data from LN biopsies from lung cancer patients. Together, our data point to a critical role for tumor-draining LNs in maintaining a stable reservoir of tumor-reactive T cells over the course of tumor development 10/
and this fits with recent data from humans and mice suggesting that immunotherapy could be acting on T cells outside of the tumor. A key question is why differentiation occurs in the tumor and not the dLN, we show data to support that T cells are 11/
exposed to persistent TCR signals in the tumor, but not the dLN. This with the TCR motif data leads us to hypothesize that the LN itself has an important role in serving as a site that protects T cells from antigen exposure, thus preventing loss of high avidity tumor-specific 12/
CD8 T cell clones. The immunobiology of T cells in tumor dLNs has not been well studied and we think that our findings could point to a novel therapeutic entry point: targeting T cells in the tumor draining LN. Further, the fact that dLN T cells are similar btwn 13/
dLNs associated with hot and cold tumors suggests that the cold tumor phenotype may not be driven by global T cell exhaustion. Moreover, it suggests patients with cold tumors could have the T cells they need for therapeutic responses sitting in their tumor-draining LNs. 14/n

 Read on Twitter
Read on Twitter