thread
EU VACCINE SUPPLY
1. If, as seems likely, the AstraZeneca/EU Advanced Purchase Agreement (APA) is similar to the Curevac/EU APA, AstraZeneca's legal position in regard to delay and/or reduction of supply appears strong.
EU VACCINE SUPPLY
1. If, as seems likely, the AstraZeneca/EU Advanced Purchase Agreement (APA) is similar to the Curevac/EU APA, AstraZeneca's legal position in regard to delay and/or reduction of supply appears strong.
2. The APA can be found here in redacted format:
https://ec.europa.eu/info/sites/info/files/curevac_-_redacted_advance_purchase_agreement_0.pdf
https://ec.europa.eu/info/sites/info/files/curevac_-_redacted_advance_purchase_agreement_0.pdf
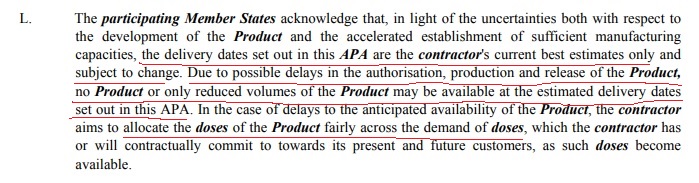
3. The Recitals makes general acknowledgement for possible changes to: current best estimates, reduced volumes & possible delays.
4. In the body of the APA at 1.12 DELAYS includes provision for delay caused by lack of EMA authorisation.
5. At 11.4 TERMINATION OF THE APA, the pharmaceutical firm may terminate the agreement if EMA authorisation isn't granted. The EU Commission has 30 days to object.
To date EMA authorisation hasn't been granted. Maybe AZ are losing patience & have threatened to terminate?
To date EMA authorisation hasn't been granted. Maybe AZ are losing patience & have threatened to terminate?
6. I skimmed through other sections, noting the vaccine documentation requirement was in English.
There's been rumour that the EU wanted AZ to translate the documents into the various languages of the EU 27, but if this APA is anything to go by, there's no legal requirement.
There's been rumour that the EU wanted AZ to translate the documents into the various languages of the EU 27, but if this APA is anything to go by, there's no legal requirement.
7. It appears to me that if the AZ agreement is similar to the Curevac APA, the EU's position is... a bit bizarre.
The EU have asked AZ for sight of its order book to compare order fulfillment to other parties.
The EU have asked AZ for sight of its order book to compare order fulfillment to other parties.
8. I imagine this has been refused by AZ on the grounds of confidentiality, so the EU has set about on a smear campaign.
It's extraordinary behaviour. Threatening to prevent vaccine exports from AZ's European facility & making claims about poor efficacy in over 65's.
It's extraordinary behaviour. Threatening to prevent vaccine exports from AZ's European facility & making claims about poor efficacy in over 65's.
9. Will take a longer look at the APA tomorrow.
10. Missed this last night. 1.3 SUBJECT MATTER. Best efforts for production & supply kick in ONCE (at least) EMA conditional approval granted. To date, approval hasn't been granted, so obligations aren't (yet) enforceable.
13. To be clear, the remarks re the 8% efficacy were purportedly from German officials/experts. If AZ contract contains a similar provision, vaccine supply could in theory be withheld to Germany on the basis they have undermined the Oxford vaccine via leaks to reporters/media.
14. The EU's stance in preventing member states ordering extra vaccines outside of the EU's scheme appears at odds with 1.7.4. which provides member states may order additional supplies under separate agreement, provided such orders don't impede the existing APA.
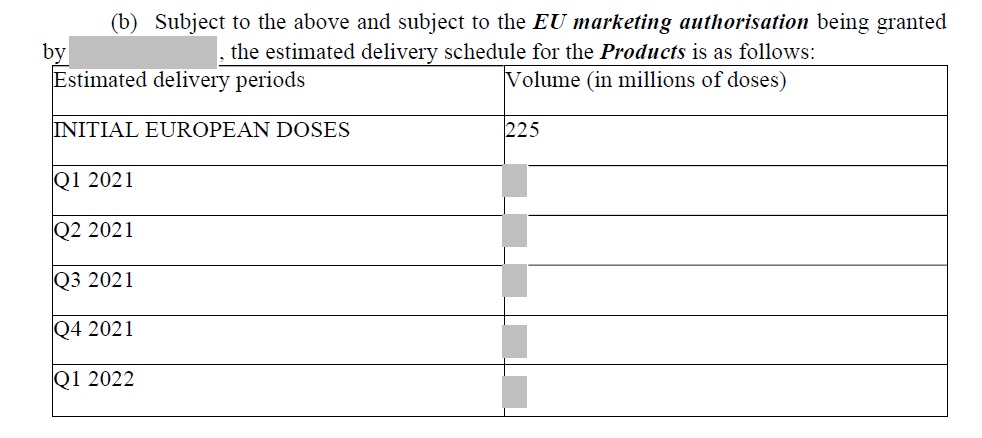
15. Strange that the EU are requesting sight of AZ's order book to elicit information on supplies to third countries, yet they've redacted their own supply schedule contained in the APA.
16. Here's the labelling requirements previously mentioned. Additional delivery time to be allowed for modification of labelling to individual ms requirements.
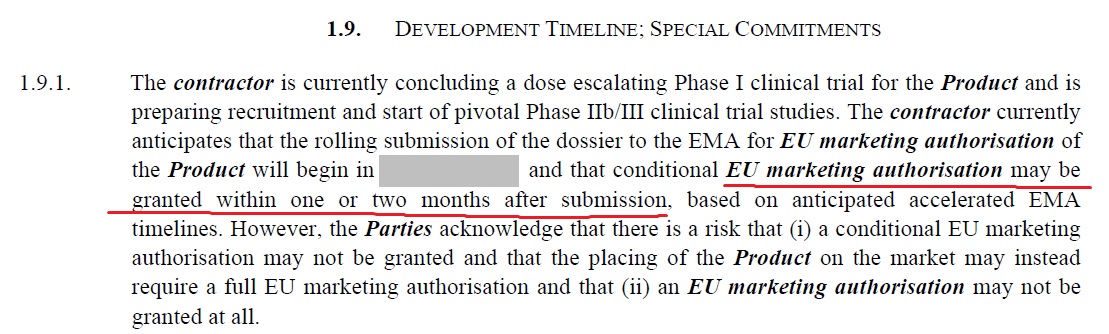
17. Here the basis of timely fulfillment of the contractual obligations is contingent on EMA conditional approval within 1 - 2 months of data submission by the pharma firm.
18. Should be remembered this isn't the AZ/EU APA, but it will be similar. This is a new area of competence (healthcare) for the EU, so the pharma firms will have taken the lead on the drafting (in my opinion) as it's their area of expertise.

 Read on Twitter
Read on Twitter