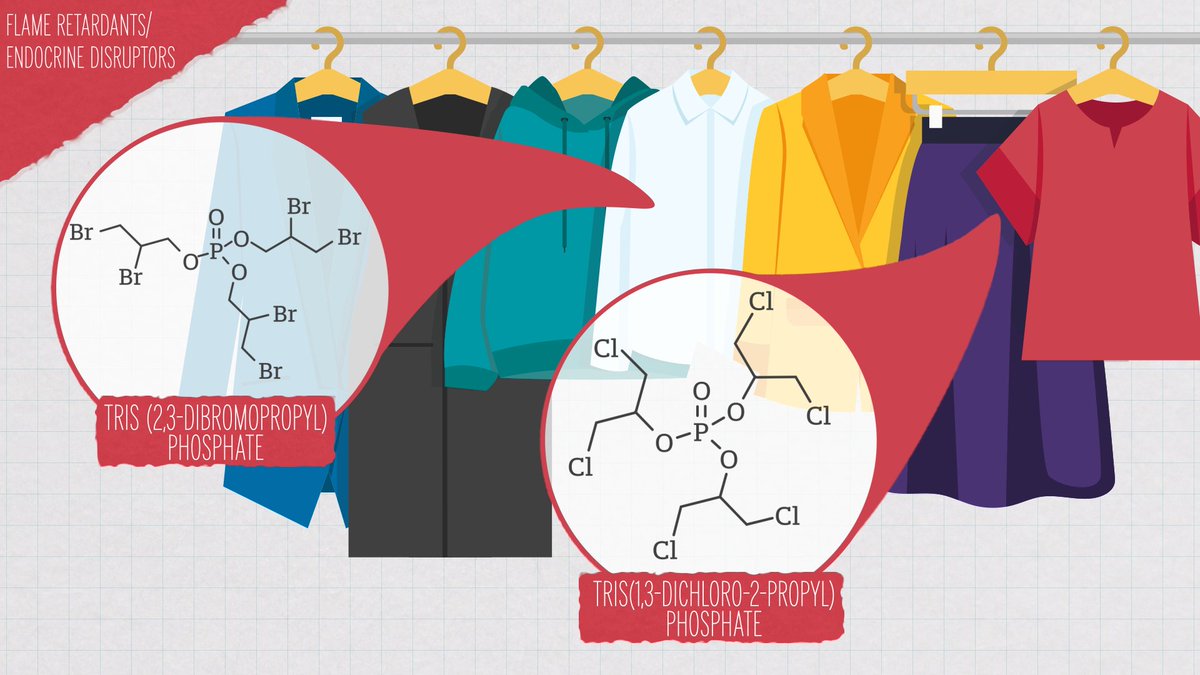
In North America, flame retardants are commonly added into clothing, furniture, electronics and children's products like car seats and cribs.
Most are organohalogens, carbon-based molecules manufactured with multiple bonds to halogen elements like bromine or chlorine.
Most are organohalogens, carbon-based molecules manufactured with multiple bonds to halogen elements like bromine or chlorine.
The retardants are true to their name in that they slow a fire rather than stopping it. In the flames, atmospheric oxygen molecules in the room break into free oxygen radicals. Radicals are molecules with lonely, unpaired electrons looking to form a bond.
Normally, they find that bond in hydrogen atoms in the hydrocarbon fuel—like in a couch or shirt. One product of this reaction is a hydroxyl radical, which then propagates the chain reaction until hydrogen atoms in the fuel source are all used up.
But let's say the couch contains a common organohalogen flame retardant called polybrominated biphenyl ester, or PBDE. When heated, PBDE gives off hydrogen bromide.
This molecule reacts with the hydroxyl radicals in the flames and leaves a bromine radical. Because bromine is more stable in radical form than oxygen, these bromine radicals do not react as easily, slowing down the entire combustion reaction.

 Read on Twitter
Read on Twitter