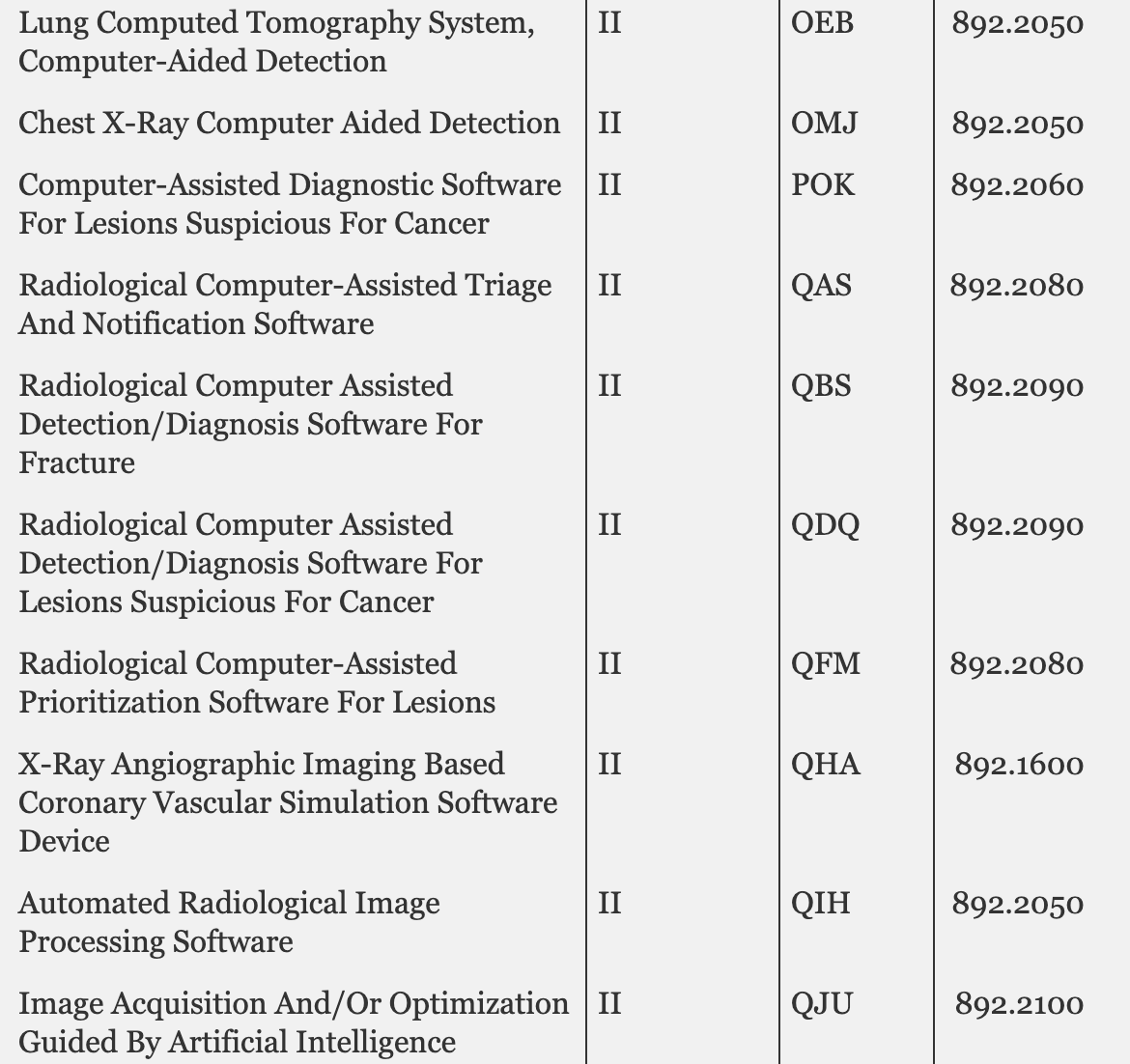
This is fairly unprecedented - the @US_FDA is considering waiving 510(k) regulatory approval for 84 types of class II medical devices including these AI-driven ones:
https://www.federalregister.gov/documents/2021/01/15/2021-00787/making-permanent-regulatory-flexibilities-provided-during-the-covid-19-public-health-emergency-by
https://www.federalregister.gov/documents/2021/01/15/2021-00787/making-permanent-regulatory-flexibilities-provided-during-the-covid-19-public-health-emergency-by
They justify this by laying out the AVERAGE costs for PMA and 510(k) approvals - start-ups take note...
The FDA propose this money could be better spent on R&D rather than expensive regulatory processes.
The FDA propose this money could be better spent on R&D rather than expensive regulatory processes.
But strangely they also justify this on a lack of reported adverse events in the MAUDE database.
This is the point that concerns me - radiology AI has barely hit the market, so of course there aren't many safety reports yet!
This is the point that concerns me - radiology AI has barely hit the market, so of course there aren't many safety reports yet!
The last reason they justify this with is their lack of capacity to handle multiple 510(k)s at present due to the pandemic.
I don't think this justifies PERMANENT exemption for these devices.
I don't think this justifies PERMANENT exemption for these devices.
So, my reaction is that this proposal is extremely dangerous and short-sighted.
We cannot allow unregulated AI to make clinical decisions.
We cannot allow unregulated AI to make clinical decisions.
At no point in this proposal do they mention the ethical issues surrounding AI - they have taken a purely bureaucratic stance, and this will be the undoing of it.
You can (and should) leave your comments on this proposal here
https://www.regulations.gov/document?D=FDA_FRDOC_0001-10479
https://www.regulations.gov/document?D=FDA_FRDOC_0001-10479
Although for some reason it says 'comment period closed' despite being published yesterday? @RegulationsGov

 Read on Twitter
Read on Twitter