#COVID19Vaccine #India
The vaccination drive for COVID19 will soon begin in India.
Things to know:
 Why get vaccinated?
Why get vaccinated?
 Misconceptions
Misconceptions
 Vaccines In India
Vaccines In India
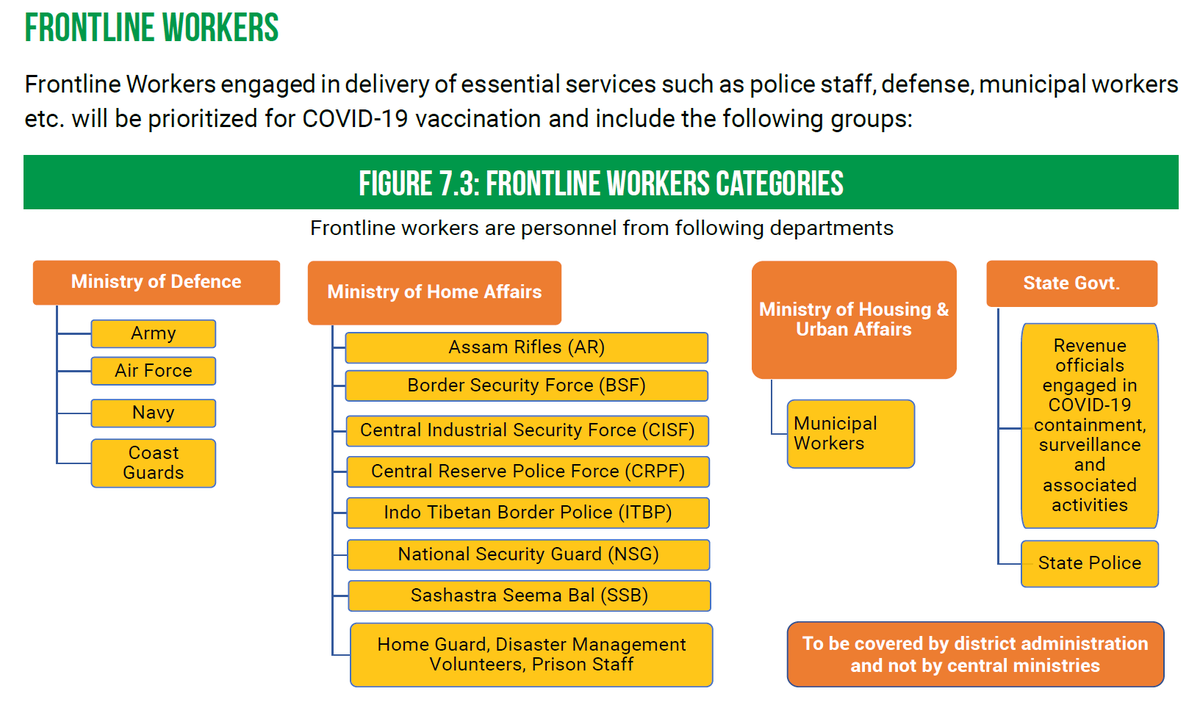
 Vaccine Priority
Vaccine Priority
 Process of Vaccination
Process of Vaccination
 Co-WIN website/app
Co-WIN website/app
 Side-effects/AEFI
Side-effects/AEFI
 Communication
Communication
1/n
The vaccination drive for COVID19 will soon begin in India.
Things to know:
 Why get vaccinated?
Why get vaccinated? Misconceptions
Misconceptions Vaccines In India
Vaccines In India Vaccine Priority
Vaccine Priority Process of Vaccination
Process of Vaccination Co-WIN website/app
Co-WIN website/app Side-effects/AEFI
Side-effects/AEFI Communication
Communication1/n
 Why Get Vaccinated?
Why Get Vaccinated?>Prevention of COVID19 infection.
>Prevention of the long term health risks of COVID19 infection.
>92 million cases and 1.9 million deaths due to COVID by now.
>Vaccine + Masks and Distancing are essential to curb the pandemic.
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html
2/n
 Misconceptions
Misconceptions>Vaccines will NOT cause infection.
>You cannot compare the 98-99% recovery rate with 95% vaccine efficacy. If you still want to - understand this: Vaccine reduces chances of mortality from 1% to 0.05%.
>Vaccines will NOT cause any mutations in your body.
3/n
>Vaccine Hesitancy needs to be addressed.
>It can stem from lack of correct information/understanding, mistrust, apprehension of side effects, propaganda by anti-vaxxers, etc.
Here's what the @MoHFW_INDIA has to say:
4/n
>It can stem from lack of correct information/understanding, mistrust, apprehension of side effects, propaganda by anti-vaxxers, etc.
Here's what the @MoHFW_INDIA has to say:
4/n
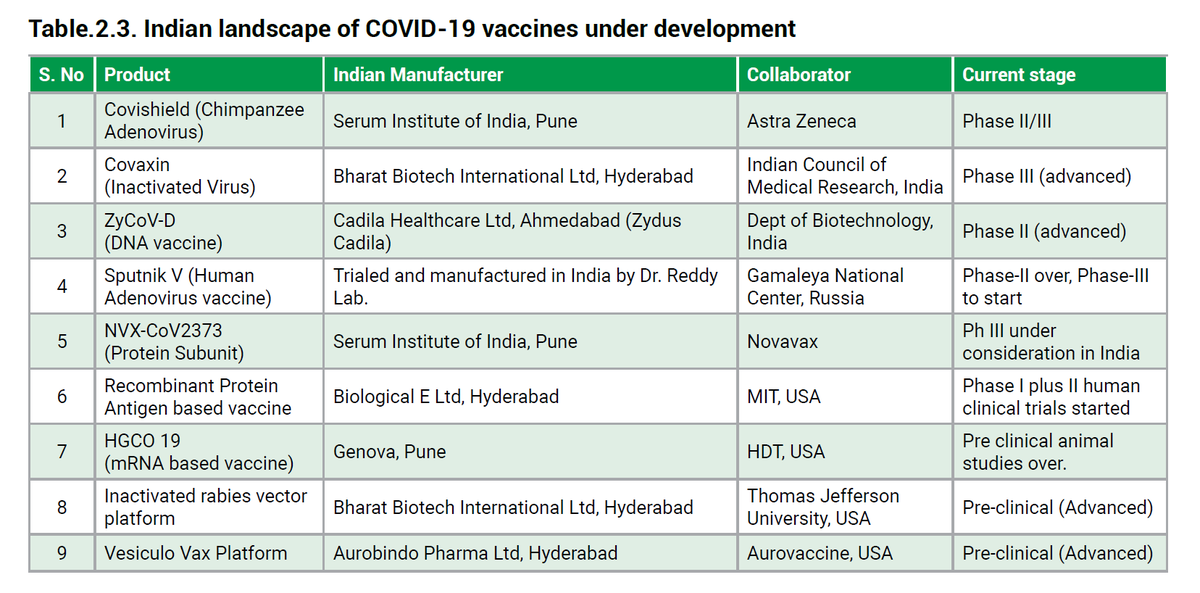
 Vaccines in India
Vaccines in IndiaThis is a list of all the vaccines undergoing development or trials in India as of 28th December 2020.
As of today, 2 of these vaccines have been approved.*
5/n
 Vaccines in India
Vaccines in India>APPROVED
1. COVISHIELD by Serum Institute of India/AstraZeneca
2. COVAXIN* by Bharat Biotech/ICMR
*approval in "Clinical Trial Mode" based on Phase 1/2 data. Phase 3 trials ongoing.
6/n
>SII has uploaded the detailed Package Insert and Fact Sheet on their website that contains all the information you need to know about COVISHIELD.
You can access those here:
Package Insert
https://www.seruminstitute.com/pdf/covishield_ChAdOx1_nCoV19_corona_virus_vaccine_insert.pdf
Fact Sheet
https://www.seruminstitute.com/pdf/covishield_fact_sheet.pdf
7/n
You can access those here:
Package Insert
https://www.seruminstitute.com/pdf/covishield_ChAdOx1_nCoV19_corona_virus_vaccine_insert.pdf
Fact Sheet
https://www.seruminstitute.com/pdf/covishield_fact_sheet.pdf
7/n
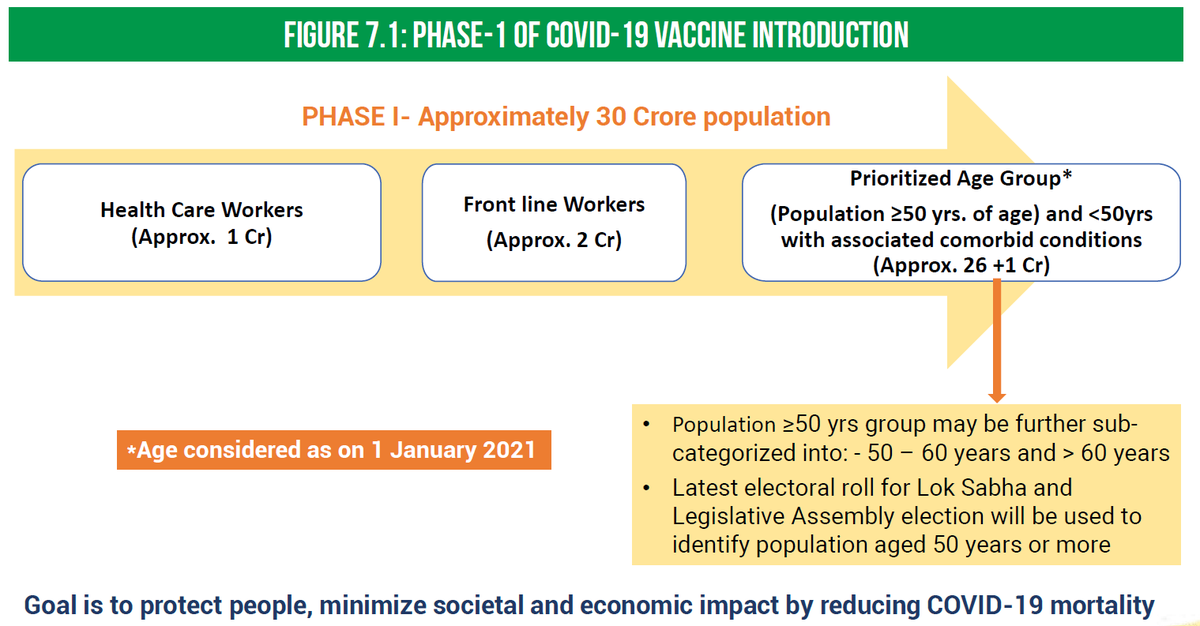
 Vaccine Priority
Vaccine Priority>The MoHFW has laid out a priority list for the 1st phase of vaccinations that includes:
1. Healthcare workers
2. Frontline workers
3. Elderly population >50 yrs of age and those <50 yrs of age with comorbidities.
https://www.mohfw.gov.in/pdf/COVID19VaccineOG111Chapter16.pdf
8/n
Guidance and instructions for the next phase will be announced in due course by the National Expert Group on Vaccine Administration for COVID-19 (NEGVAC).
Meanwhile, here's an information sheet from the MoHFW for those <50 yrs of age.
https://www.mohfw.gov.in/pdf/Covid19CommunicationStrategy2020.pdf
9/n
Meanwhile, here's an information sheet from the MoHFW for those <50 yrs of age.
https://www.mohfw.gov.in/pdf/Covid19CommunicationStrategy2020.pdf
9/n
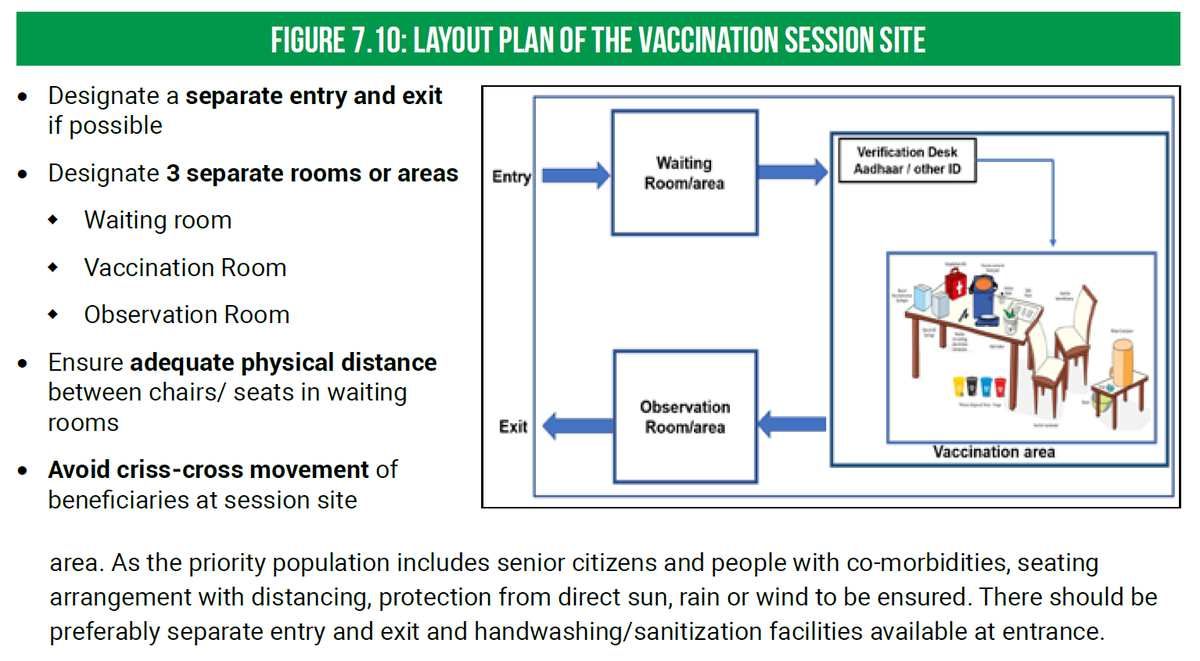
 Process of Vaccination
Process of VaccinationSteps:
1. Registration* of the beneficiary on the Co-WIN app.
2. Verification of photo ID proof at the vaccination site with that uploaded on the app.
3. Vaccination.
4. 30 mins observation
*Registration is mandatory for getting vaccinated.
10/n
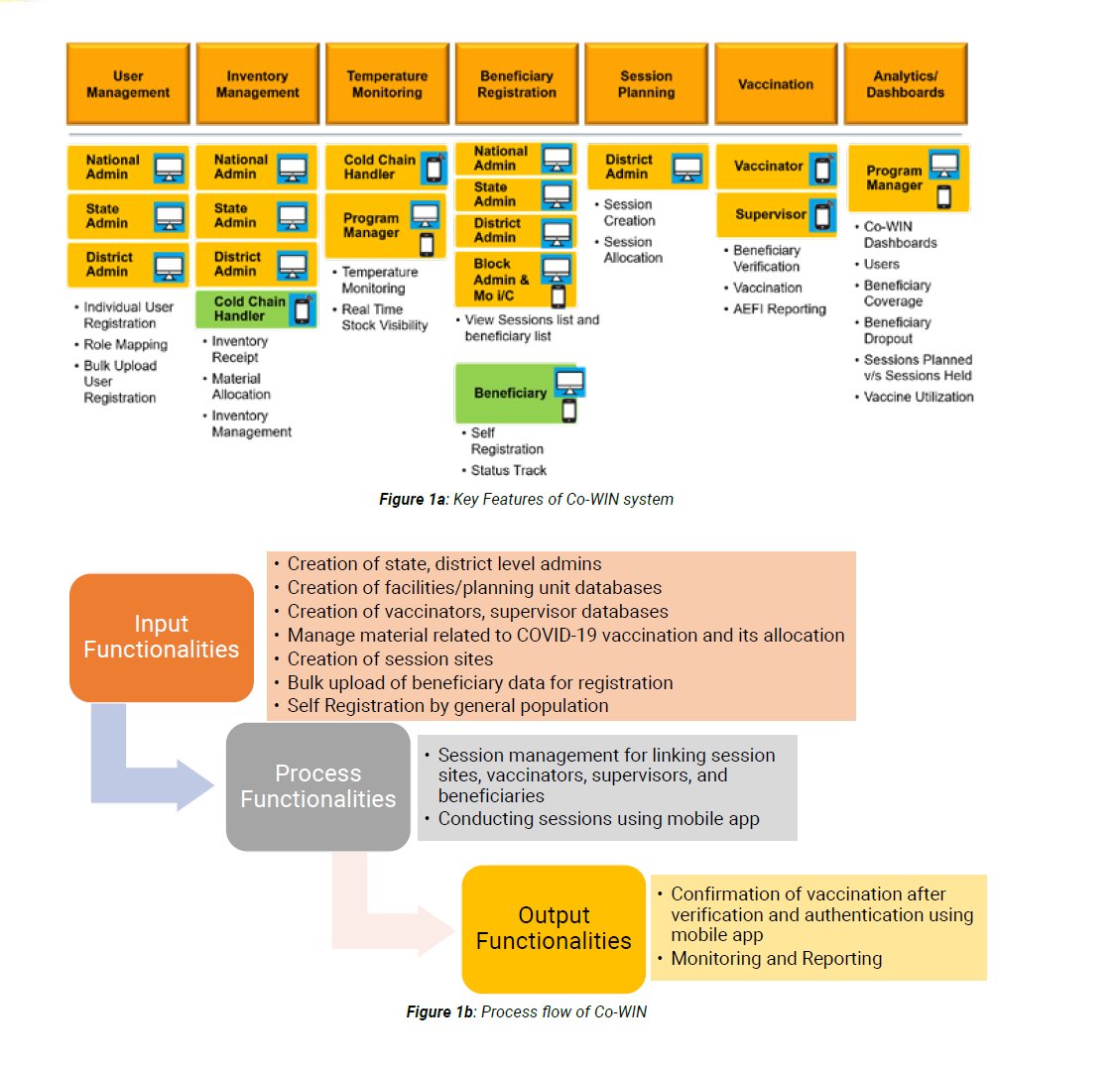
 Co-WIN website/app
Co-WIN website/app>Co-WIN is an extension of the existing electronic Vaccine Intelligence Network (e-VIN) - made specifically for COVID vaccination.
>It has 2 modules - Website and App.
>The app is under development and will soon be available for download.
11/n
FEATURES:
While Co-WIN has extensive functionalities for the authorities, it has few important uses for the beneficiaries:
> Info about vaccination
> Self-registration for vaccination
> Track status of vaccination
> Report AEFI
12/n
While Co-WIN has extensive functionalities for the authorities, it has few important uses for the beneficiaries:
> Info about vaccination
> Self-registration for vaccination
> Track status of vaccination
> Report AEFI
12/n
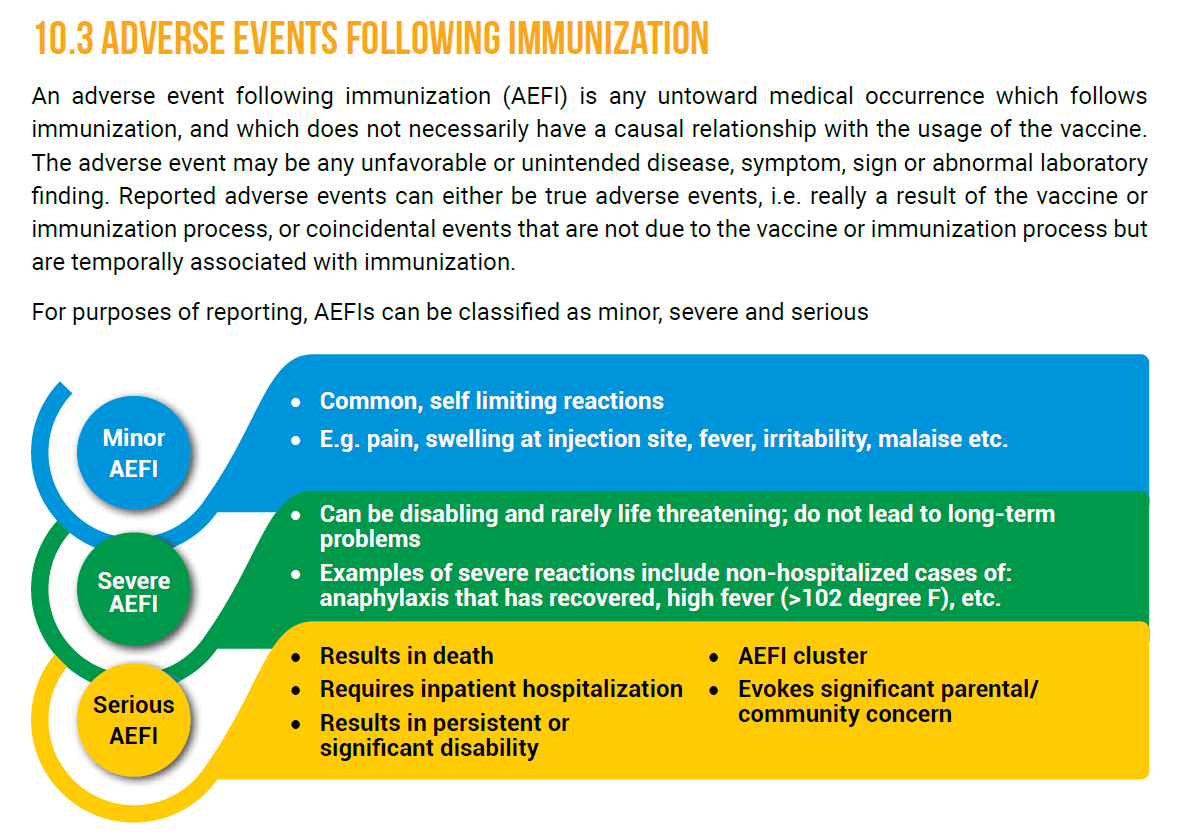
 Side effects/AEFI
Side effects/AEFIProbably the single largest concern responsible for Vaccine Hesitancy is the fear/apprehension of Side Effects or Adverse Events Following Immunization (AEFI).
13/n
 What should you expect?
What should you expect?>Mild side effects are common and expected to occur and they are self-resolving. You need not worry about those.
>Adverse events like allergic reactions/anaphylaxis are much rare. They DO NOT occur in all recipients.
14/n
 AEFI Reporting
AEFI Reporting> You will be observed for 30 mins after vaccination to look for and manage any immediate severe reactions like anaphylaxis.
>In the following days, you must report any other symptoms/discomfort to the authorities and get investigated.
15/n
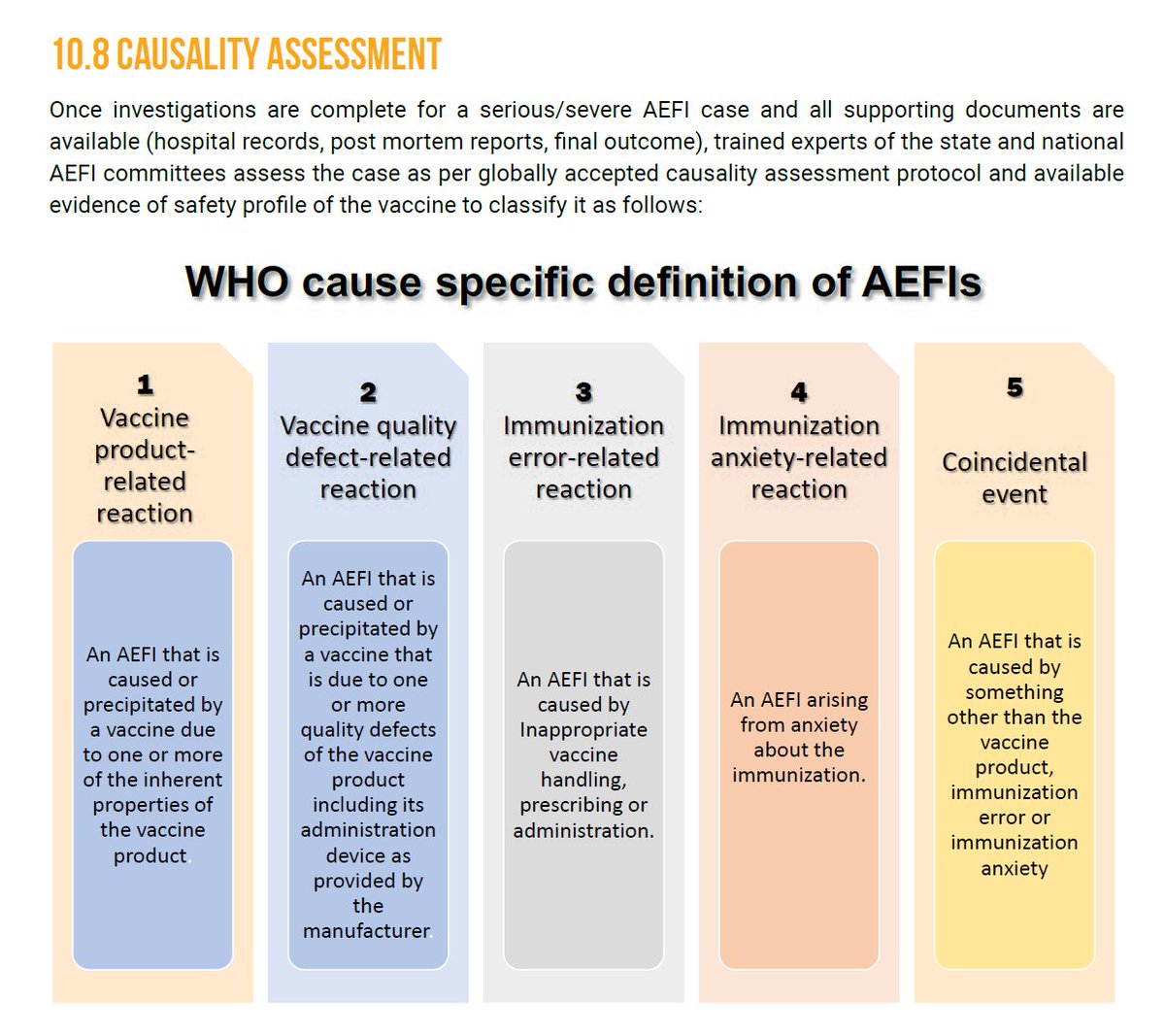
 AEFI Investigation
AEFI Investigation>AEFI are investigated and then assessed as per the WHO guidelines to determine the cause.
>It is important to understand whether it is vaccine-related or not.
>Many times, AEFI can occur due to other reasons as well, as shown in the image.
16/n
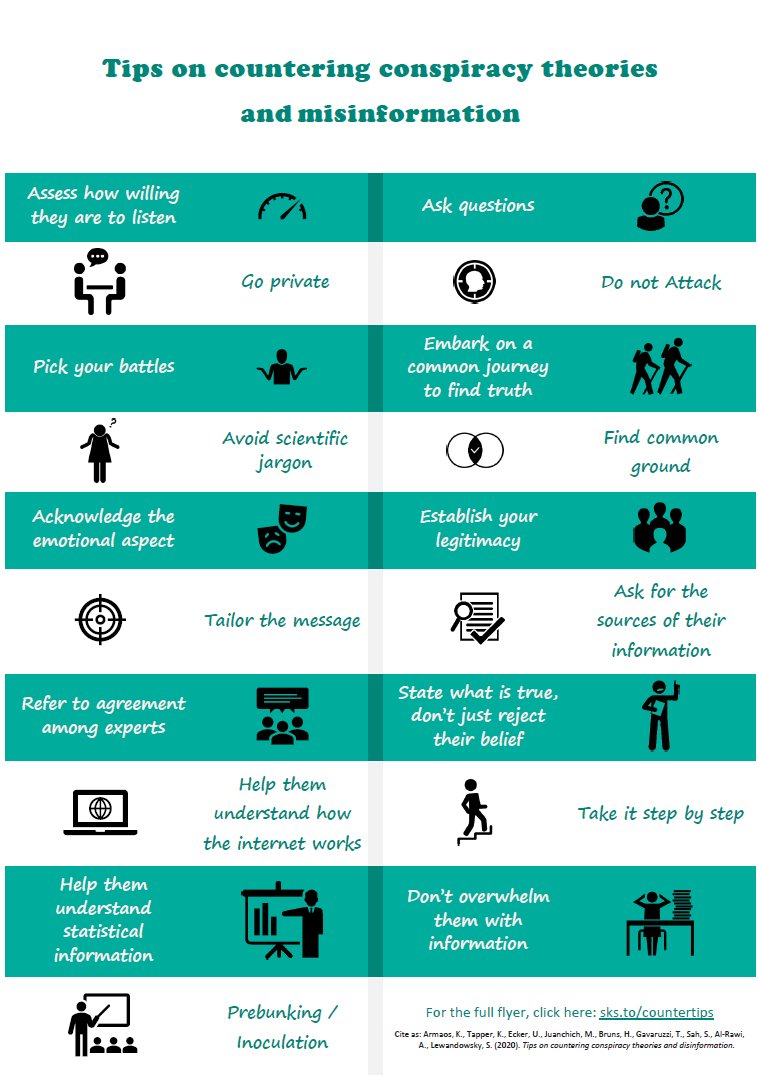
 Communication
Communication>Essential to spread correct and reliable information about vaccines.
>Also to counter misinformation spread by fake experts or anti-vaxxers.
Here is an excellent handbook developed by Prof @STWorg and Prof @adamhfinn.
https://hackmd.io/@scibehC19vax/home
17/n
 To Conclude:
To Conclude:>Vaccination will shortly begin in India.
>Registration on Co-WIN is mandatory.
>Vaccines are safe - with common, mild side effects.
>Adverse events, if any - must be reported.
>Guard against misinformation and educate others with correct, reliable info.
18/18

 Read on Twitter
Read on Twitter