Dear phage enthusiasts, scientists, clinicians, gather around. I'm @NandoGordillo and I'm thrilled to share with you our most recent paper, now out on @NatureMicrobiol. We have devised a way to use phages to revert antimicrobial resistance in A. baumannii! https://www.nature.com/articles/s41564-020-00830-7
Why A. baumannii? Because it's one of the biggest threats when it comes to antimicrobial resistance. It's been deemed THE top priority for new therapeutic strategies. And senior author @JeremyJBarr already knew a thing or two about phage therapy against it https://aac.asm.org/content/61/10/e00954-17.abstract
Among its many weapons, I need to highlight the capsule that A. baumannii produces. It's a complex, viscous layer of sugars. It is produced by the "k" locus, and defends A. baumannii against threats such as antibiotics and the immune system. SEM pic here:
Our lab regularly receives sewage samples from all over Australia. This project began isolating and characterising phages against a panel of A. baumannii strains. We highlight two phages: FG02 (after yours truly) and CO01 (after Cody Oliveira, brilliant undergrad).
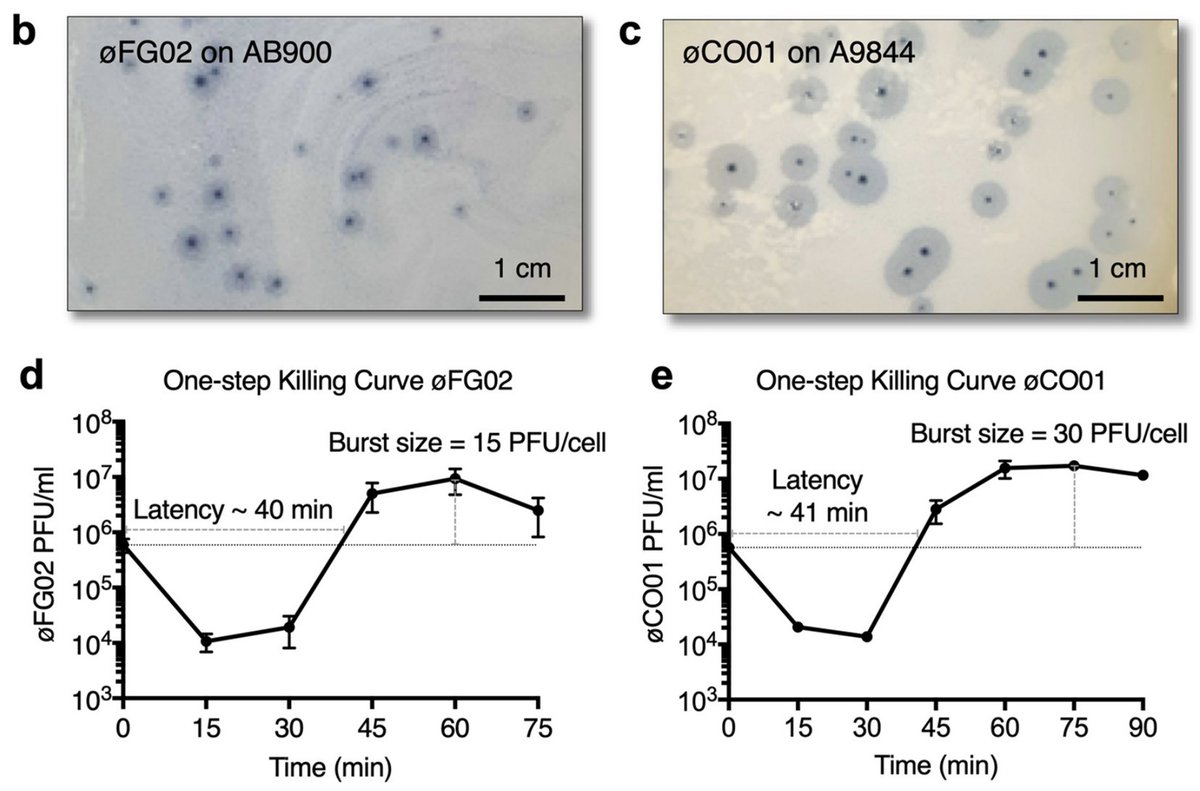
On their hosts, these phages produce beautiful plaques with a clear center and a hazy halo. We described their one-step killing curve, host range and a brief overview of their genetic composition.
And then, the inevitable happened. After a few hours of co-incubation, A. baumannii became resistant to our phages. So we wondered what was the mechanism it had used to become phage-resistant.
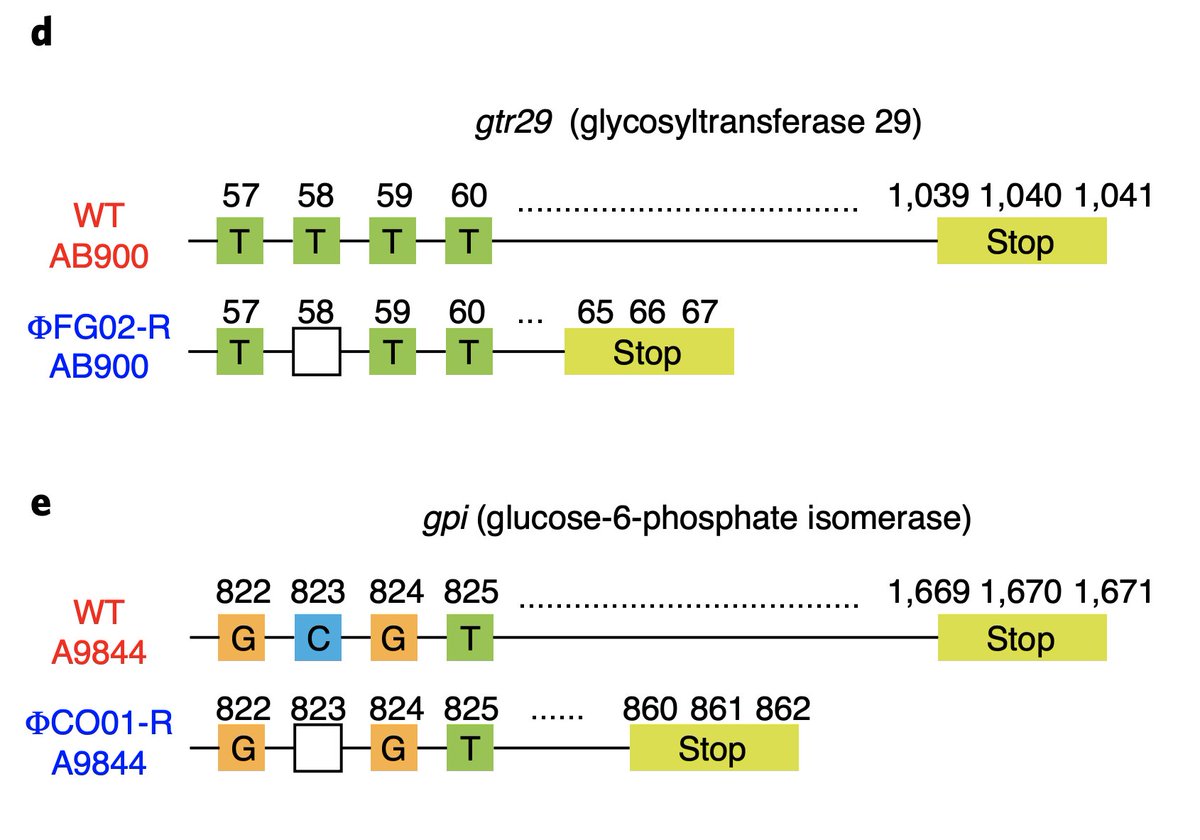
We sequenced the genomes of the wild type strains and the phage-resistant mutants; and compared them (big shoutout to Stuart Archer from @MonashBioinfo!). We discovered that the phage-resistant mutants had loss-of-function mutations in genes of the "k" locus.
As a result, phage-resistant A. baumannii was not producing its capsule! This SEM picture (by Denis Korneev) is probably my favourite from the study. On the left, you see the original, capsulated A. baumannii. On the right, phage-resistant A. baumannii without capsule.
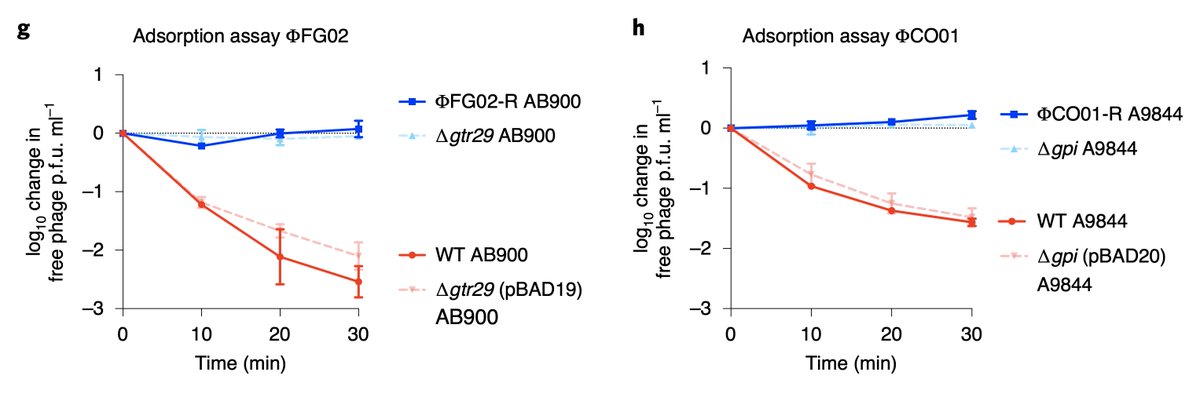
We knocked out those k-locus genes to confirm they were responsible for phage-resistance. We also performed an adsorption assay and confirmed that our phages could not attach to the phage-resistant isolates.
The capsule of A. baumannii is the receptor of phages FG02 and CO01.
The capsule of A. baumannii is the receptor of phages FG02 and CO01.
And this is where things got VERY exciting! Phage-resistant A. baumannii had lost its capsule, so we expected to find some associated trade-offs. After all, we knew that A. baumannii depends on its capsule for many things.
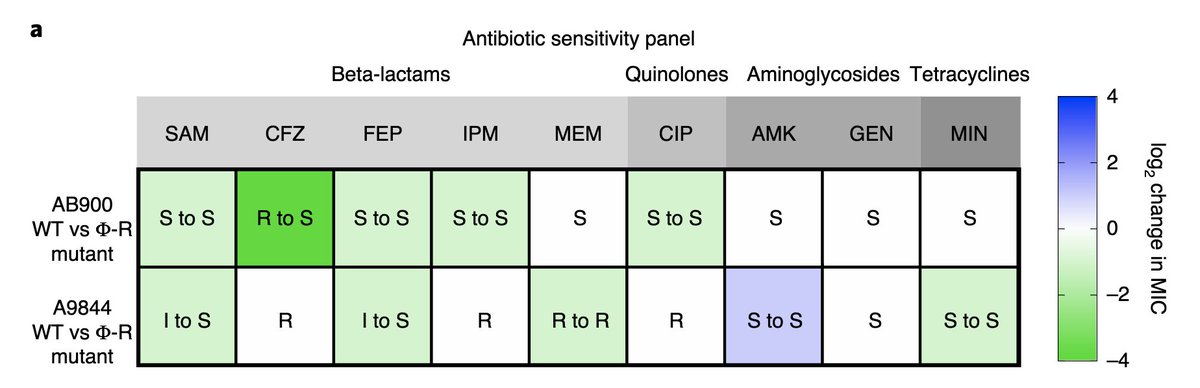
First and foremost: we observed resensitization to antibiotics 
 . 7 out of 9 tested antibiotics were more effective against phage-resistant A. baumannii than against wild type.
. 7 out of 9 tested antibiotics were more effective against phage-resistant A. baumannii than against wild type.

 . 7 out of 9 tested antibiotics were more effective against phage-resistant A. baumannii than against wild type.
. 7 out of 9 tested antibiotics were more effective against phage-resistant A. baumannii than against wild type.
But that was just one of phage-resistant A. baumannii's problems:
- It had become resensitized to the human complement system.
- It could be killed by other phages it used to resist.
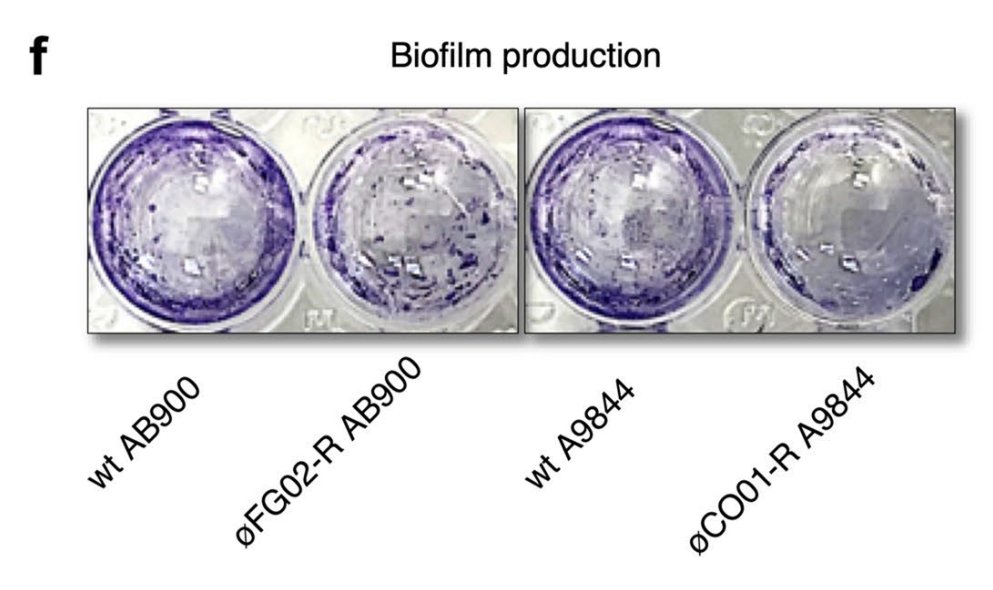
- It could not form biofilms.
- It was less fit in a mouse model of bacteremia.
- It had become resensitized to the human complement system.
- It could be killed by other phages it used to resist.
- It could not form biofilms.
- It was less fit in a mouse model of bacteremia.
By giving up its capsule, trying to escape from our phages, A. baumannii had backed itself into a corner.
We forced it into a dilemma: death by phage or death by antibiotics.
We forced it into a dilemma: death by phage or death by antibiotics.
I am currently working on more pre-clinical models to corroborate the effectiveness of this phage therapy approach against A. baumannii. Watch this space, current data is very encouraging.
Phage therapy is a versatile ally in our fight against antimicrobial resistance.
Phage therapy is a versatile ally in our fight against antimicrobial resistance.
This is a picture of just a few members of the amazing team that worked on this project (enjoying our Christmas picnic last month). But this is my chance to thank everyone else involved in it! @TrevorLithgow @AntonPeleg @OBryanMoira and more, not on Twitter (yet).
I hope you enjoyed my little rant. Chuck us a RT on the first tweet of the thread and please share the phage gospel! Both @JeremyJBarr and I ( @NandoGordillo) will be more than happy to elaborate on any of the points!
Gracias
#phage #phagetherapy #superbugs #amr
Gracias

#phage #phagetherapy #superbugs #amr

 Read on Twitter
Read on Twitter