New preprint on the fascinating KdpFABC K+ pump
 . Lead by grad student Marie Sweet and the Stokes group at NYU ( @SkirballNYUSoM), we are proud to be part of this comprehensive study on the "Structural basis for Potassium transport by KdpFABC". 1/8 https://www.biorxiv.org/content/10.1101/2021.01.09.426067v1
. Lead by grad student Marie Sweet and the Stokes group at NYU ( @SkirballNYUSoM), we are proud to be part of this comprehensive study on the "Structural basis for Potassium transport by KdpFABC". 1/8 https://www.biorxiv.org/content/10.1101/2021.01.09.426067v1

 . Lead by grad student Marie Sweet and the Stokes group at NYU ( @SkirballNYUSoM), we are proud to be part of this comprehensive study on the "Structural basis for Potassium transport by KdpFABC". 1/8 https://www.biorxiv.org/content/10.1101/2021.01.09.426067v1
. Lead by grad student Marie Sweet and the Stokes group at NYU ( @SkirballNYUSoM), we are proud to be part of this comprehensive study on the "Structural basis for Potassium transport by KdpFABC". 1/8 https://www.biorxiv.org/content/10.1101/2021.01.09.426067v1
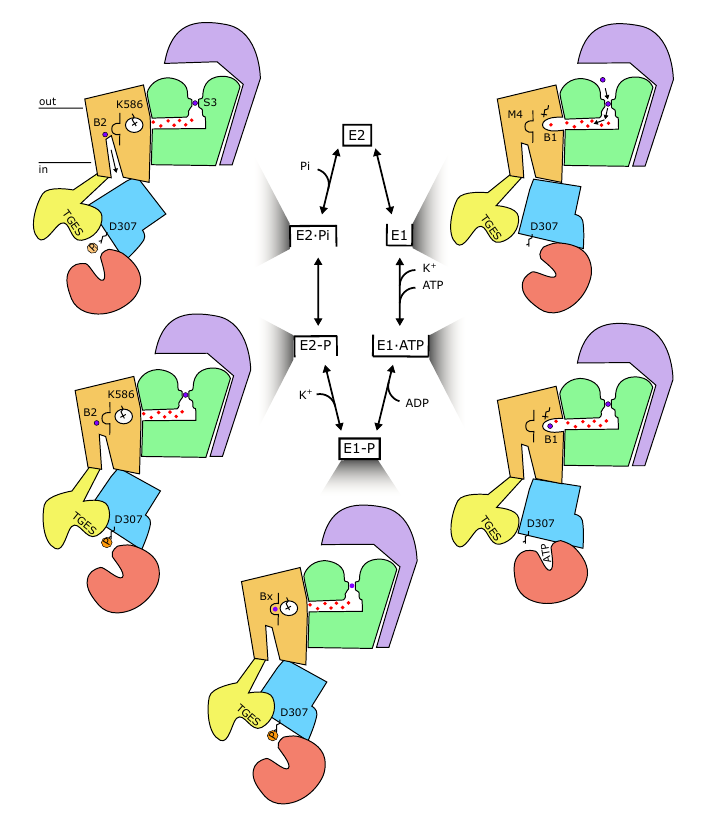
KdpFABC is an ATP-dependent K+ pump that ensures bacterial survival in K+-deficient environments. It's a unique complex between a channel-like subunit and a pump-like subunit. Recent studies on KdpFABC have led to conflicting suggestions about transport pathway and mechanism. 2/8
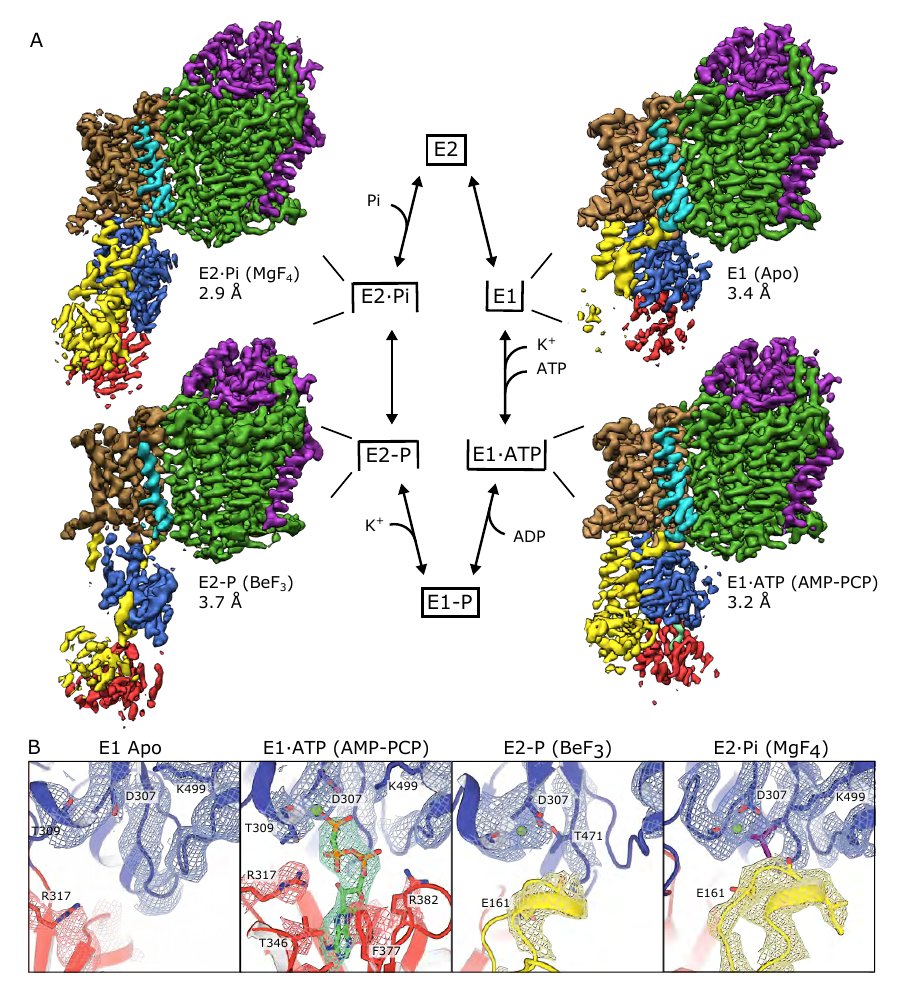
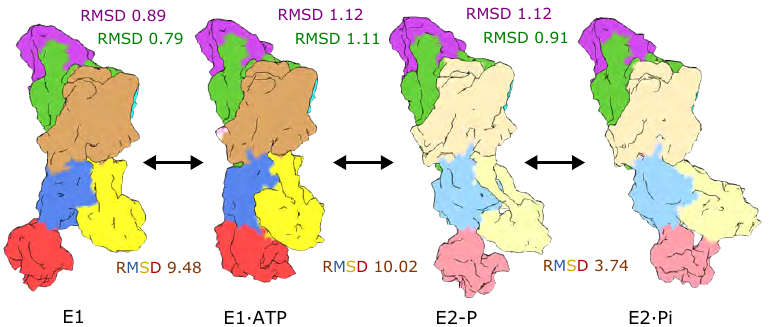
Here, we solved cryo-EM structures of KdpFABC in a range of conformations using substrate analogs: 14 maps (2.9-3.7 Å) and 5 models allows us to capture the four key states in the transport cycle. 3/8
We find that KdpB undergoes conformational changes during the transport cycle that are consistent with other representatives from the P-type pump superfamily. The other subunits, KdpA (purple) and KdpC/KdpF (green), remain static. 4/8
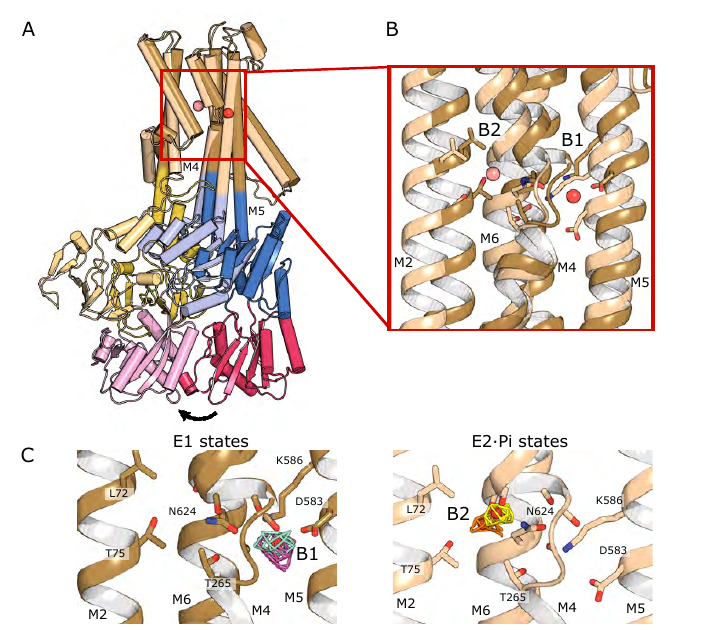
Excitingly, we observe a series of spherical densities buried in the complex that we assign as K + or water. These spherical densities define a pathway for K + transport through the complex. 5/8
In the middle of the membrane, this pathway runs through an intramembrane tunnel in KdpA and delivers ions to sites in the membrane domain of KdpB. While unchanged in KdpA, in KdpB the ion sites change depending on the conformational state! 6/8
Our structures thus suggest a mechanism supporting the "KdpA-KdpB pathway", where ATP hydrolysis is coupled to potassium transfer between alternative sites in the membrane domain of KdpB, and we can now present a comprehensive model for a unique K+ import system . 7/8
. 7/8
 . 7/8
. 7/8

 Read on Twitter
Read on Twitter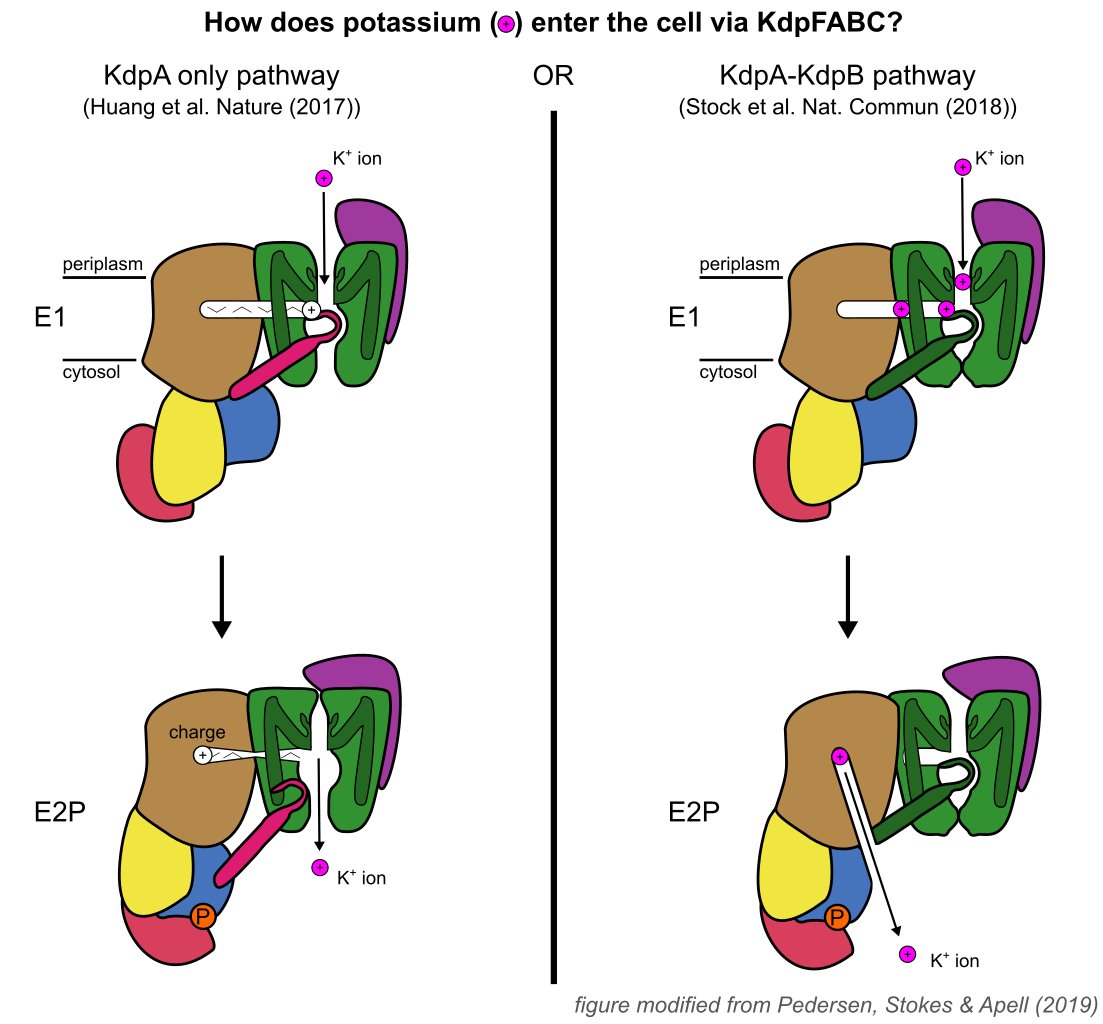


 who will defend her thesis this Friday!
who will defend her thesis this Friday!



