Today, I’d like to share information on the new drug therapy approval successes we achieved during 2020 despite the unprecedented challenges caused by the #COVID19 pandemic: https://go.usa.gov/xAnXt
In 2020, we approved 53 novel drugs never before marketed in the US, including the 1st FDA-approved medication for treating #COVID19. We also approved drugs that contain active ingredients already on the market put to new & innovative uses. https://go.usa.gov/xAnRx
58% of our novel approvals were for drugs to treat patients with rare diseases who have few or no drugs available to treat their condition. These approvals can mean new hope for an enhanced quality of life, and in some cases, increased survival.
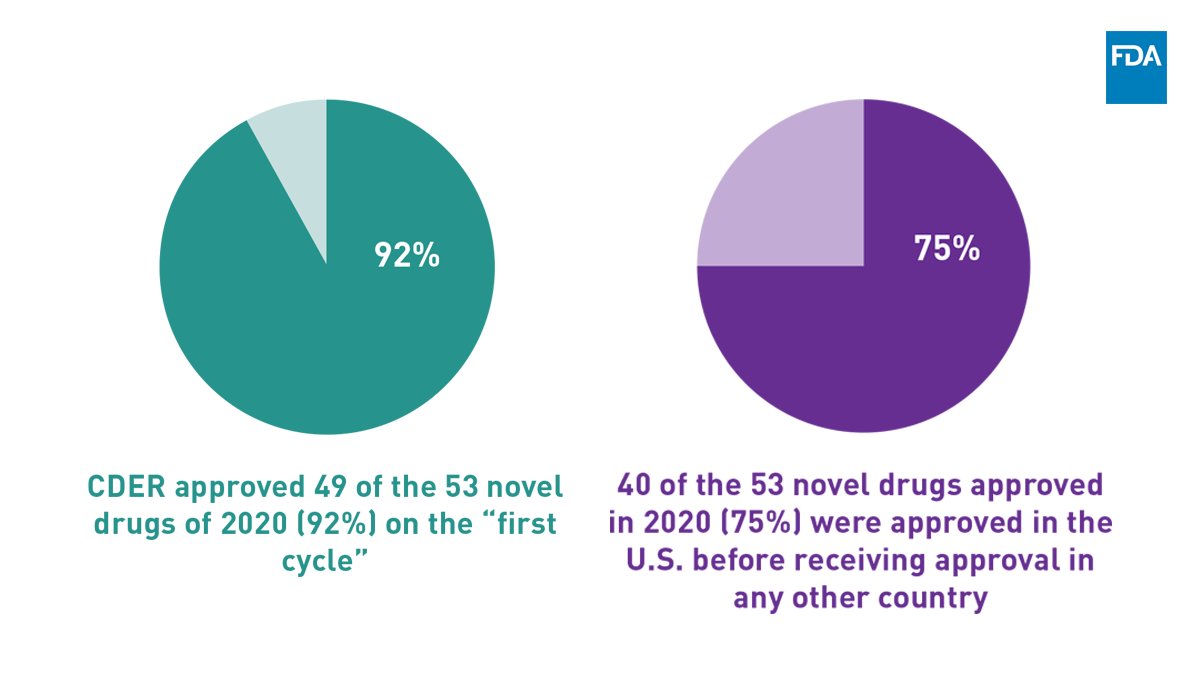
Our 2020 approvals reflect many efficiencies in our review process. CDER met its Prescription Drug User Fee Act (PDUFA) goal dates for 100% of the novel drugs approved, 92% on the first cycle. Most were approved in the U.S. before any other country in the world.
I am honored to work with so many in CDER dedicated to bringing new therapies to patients as quickly as possible, while ensuring our consistently high standards for science, safety, and efficacy. As 2021 begins, we look forward to continuing to serve the American public.

 Read on Twitter
Read on Twitter