Excited to share our recent paper in #Nature Genetics @NatureGenet. A brief #tweetorial  highlighting the key aspects on the importance of amino acid transporter #Slc7a5 for #Kras mutant #colorectalcancer #ScienceTwitter
highlighting the key aspects on the importance of amino acid transporter #Slc7a5 for #Kras mutant #colorectalcancer #ScienceTwitter
A thread (including cats&dogs ) 1/n
 highlighting the key aspects on the importance of amino acid transporter #Slc7a5 for #Kras mutant #colorectalcancer #ScienceTwitter
highlighting the key aspects on the importance of amino acid transporter #Slc7a5 for #Kras mutant #colorectalcancer #ScienceTwitter A thread (including cats&dogs ) 1/n
We’ve previously shown that #apc loss and #Kras mutation increases cell proliferation, tumor formation and can drive #resistance to #targeted therapy. However it wasn’t clear how the #Kras mutation achieved this.
Seminal works from Alec Kimmelman @nyulangone, @mvh_lab (to name a few) had previously shown that Kras mutation extensively rewires #metabolism
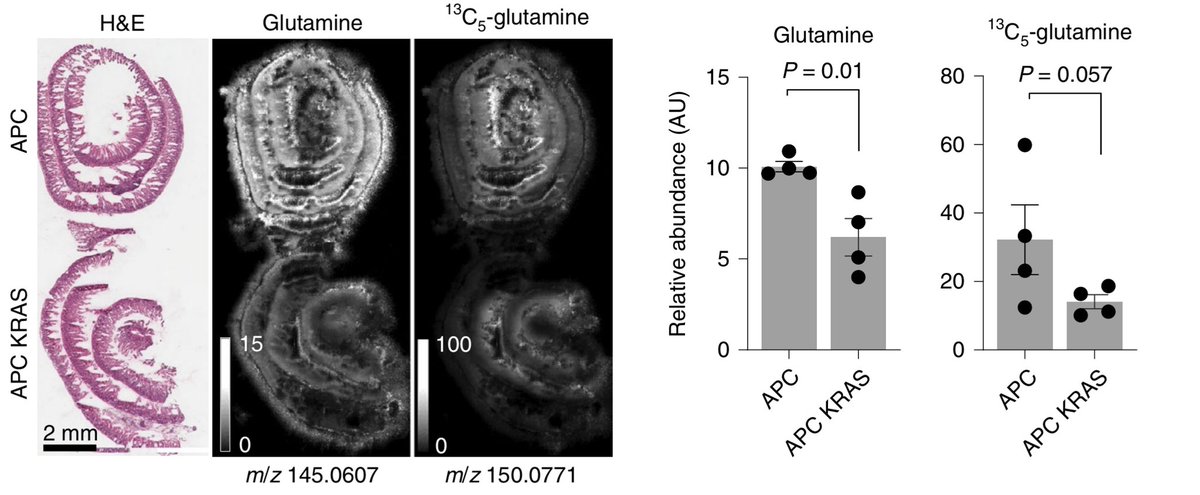
To address this, we performed #transcriptomic and #metabolomic analyses of intestinal mouse models w/ or w/o Kras mutation & found Kras mutant intestinal tissues have low glutamine levels compared to WT. This wasn’t what we expected.
The assumption was Kras activation will drive towards increased uptake of nutrients to meet the anabolic demands
This is where the #Rosetta #GrandChallenge Team @CancerGrand were crucial – together w/ them we established a mass spectrometry #imaging #msi platform to look at metabolites in situ. This confirmed lack of glutamine in Kras mutant tissues. Importantly this wasn’t due to  usage
usage
 usage
usage
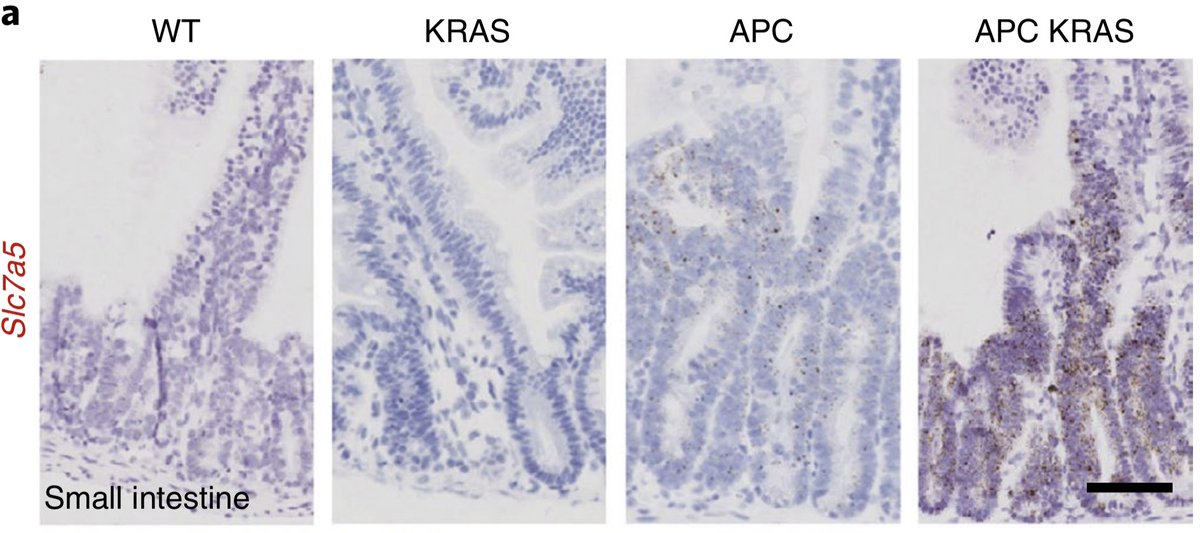
We found that Kras mutant cells upregulated #Slc7a5 an amino acid transporter - shown previously by Leon O. Murphy lab (Nicklin et al., 2009) and Muthuswamy Lab ( @3dorganoid) recently in regulating nutrient stress.
When we stained for #Slc7a5 we found it selectively upregulated on both mRNA and protein levels in #Apc deficient #Kras mutant tissues and tumours.
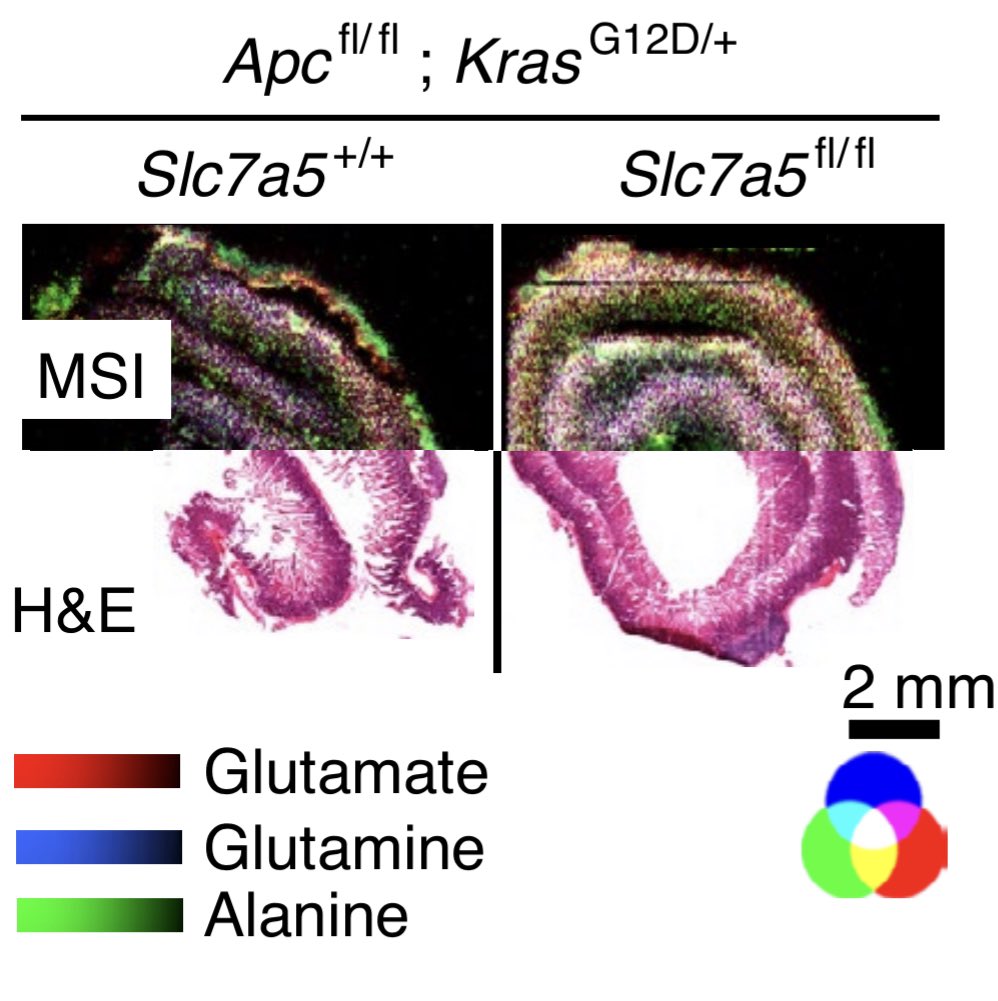
A #transmembrane antiporter #Slc7a5 effluxes glutamine from cells to import essential amino acids: its loss profoundly reduced proliferation and increased survival specifically in Kras mutant setting.
We asked whether loss of #Slc7a5 functionally changes the #amino acid levels. Indeed #Slc7a5 loss led to higher Gln accumulation, loss of labelled tyrosine and leucine shown using #PET-imaging (tyr) and #msi (leu).
But cells are able to survive #Slc7a5 loss - so we next asked what kind of trickery they have done to deal with this. It turns out they reduce #translation rates and specifically downregulate longer mRNAs and have lower mTOR signaling.
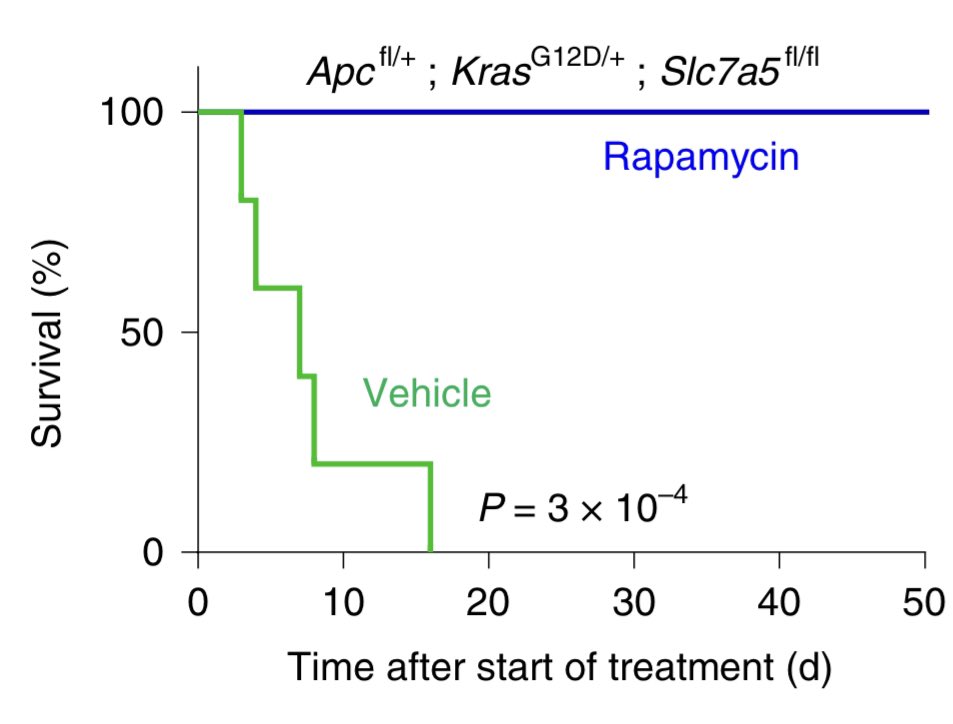
There was evidence from us (recently on @cancerdiscovery) and others showing #Kras mutant cells are resistant to #mTOR inhibition. We wondered whether losing #Slc7a5 would now make these cells sensitive
Importantly, losing #Slc7a5 made cells sensitive to #mTOR inhibition leading to reduced tumour cell proliferation, signalling and markedly improved survival.
Next we asked whether these findings will be applicable to the human setting. Excellent collab with @DrPipDunne @MallSid, suggested #Slc7a5 as a strong prognostic indicator for #Kras mutant #CRC.
#CRC #metastasis is the most common cause of mortality. @jackstadt_lab during his time in Glasgow had developed the #autochthonous #metastatic #KPN mouse model. Using this model we saw loss of #Slc7a5 not only improved survival but also reduced metastasis.
We think #Slc7a5 is an important therapeutic target and potential biomarker in #Kras #CRC. The simple act of #Slc7a5 maintaining the right amino acid pools turns out to be critical for #cancer cell proliferation, survival and metastasis.
Thanks to the AMAZING #Rosetta #Grandchallenge @CancerGrand team @NPL, @ASTRAZENECAUK, ZTakats @imperialcollege & the metabolomics team @CRUK_BI, @DSumpton for pushing this work to the finish line during the first lockdown. Great collaborators Bushell Lab, @DavidYLewis and more
If you read it this far, here’s some  from us to you
from us to you
 from us to you
from us to you
Thanks to @vermeulenlouis1 lab for their excellent N&Vs on the paper 
 the schematic
the schematic
Link https://www.nature.com/articles/s41588-020-00758-y
https://www.nature.com/articles/s41588-020-00758-y

 the schematic
the schematic Link
 https://www.nature.com/articles/s41588-020-00758-y
https://www.nature.com/articles/s41588-020-00758-y

 Read on Twitter
Read on Twitter