This is a limited point about availability of efficacy data for vaccines under development in the context of the approval for CovidShield and Covaxin in India.
There have been many so-called experts on the idiotbox opining about apparent availability of P III data which 1/n
There have been many so-called experts on the idiotbox opining about apparent availability of P III data which 1/n
2/n apparently the SEC had access to based on which it "supposedly" approved Covaxin. Another argument that is prevalent is other regulators (US FDA and MHRA) also approved vaccines based on P II data alone. Let me give you a few facts so that you can make your own decision.
3/n The protocols for both mRNA vaccines are publicly available. You can check. Both protocols *define* when the interim analysis will be done. This is not subjective. They clearly define how many infections need to be documented before the Data Safety Monitoring Board meets.
4/n Find the protocols for the bridging study for CovidShield and Covaxin and look for a similar milestone.
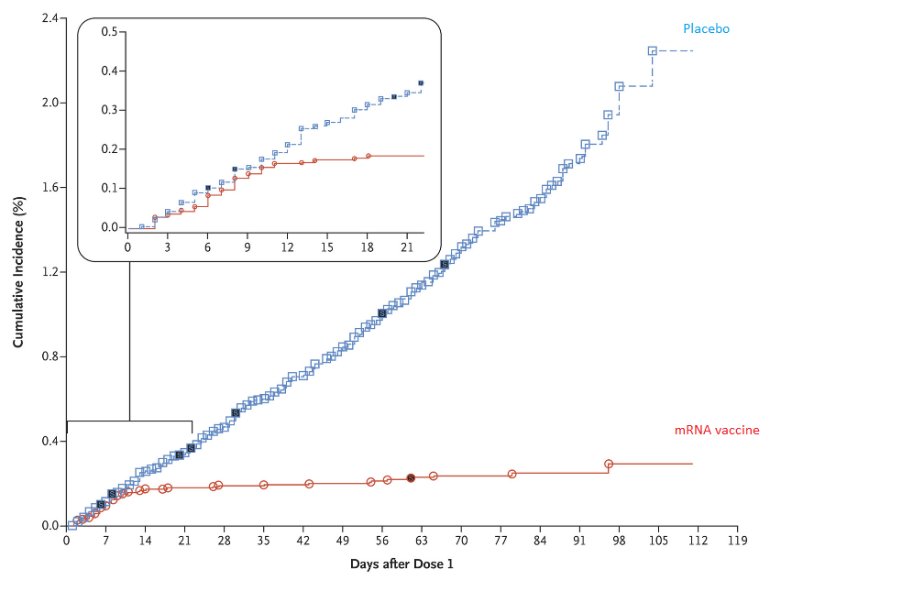
Here is one set of efficacy data post the interim analysis of a mRNA vaccine.
Source: https://www.cdc.gov/library/covid19/122220_covidupdate.html
Here is one set of efficacy data post the interim analysis of a mRNA vaccine.
Source: https://www.cdc.gov/library/covid19/122220_covidupdate.html
5/n This data was analyzed post the interim analysis where the blind was broken by the DSMB. Now ask yourself this question:
How does the SEC, or the sponsor of these studies, or the experts who are offering their opinion liberally on the idiotbox know what the efficacy is
How does the SEC, or the sponsor of these studies, or the experts who are offering their opinion liberally on the idiotbox know what the efficacy is
6/n without the blind being broken? Do they have some divine insight into how the vaccine candidates are working? On what basis are they offering their considered opinion? Is that a valid question? Or is it that asking it makes one anti-national?
7/n To those who interview these experts, please ask the following simple questions:
A. Do they know if the blind was broken for the bridging study and the Phase III study?
B. If so, can they produce data like the one above showing how many subjects who were infected were
A. Do they know if the blind was broken for the bridging study and the Phase III study?
B. If so, can they produce data like the one above showing how many subjects who were infected were
8/n on placebo Vs the vaccine candidate?
And if they cannot answer this question, then ask the following question:
C. In the absence of efficacy data, how does one claim that the vaccine candidate is effective?
D. Do they agree that therapeutic candidates ought to be approved
And if they cannot answer this question, then ask the following question:
C. In the absence of efficacy data, how does one claim that the vaccine candidate is effective?
D. Do they agree that therapeutic candidates ought to be approved
9/n just based on Phase II data? This is a yes or no question. Dont let them confuse the issue saying this is an emergency situation. Science doesnt work differently in emergencies.
If the decision is based on considerations other than Scientific, say that. Dont hide behind
If the decision is based on considerations other than Scientific, say that. Dont hide behind
10/n the integrity that Science offers and provide a fig leaf to the SEC and the DCGI.
BTW, if you want to know more, read this excellent summary from the @WHO : https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiIpKWX8YnuAhXDjVkKHZvlBvsQFjACegQIBBAC&url=https%3A%2F%2Fapps.who.int%2Firis%2Frest%2Fbitstreams%2F1324092%2Fretrieve&usg=AOvVaw2PEz1LVzsJqGVDHz1BHOJ-
BTW, if you want to know more, read this excellent summary from the @WHO : https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiIpKWX8YnuAhXDjVkKHZvlBvsQFjACegQIBBAC&url=https%3A%2F%2Fapps.who.int%2Firis%2Frest%2Fbitstreams%2F1324092%2Fretrieve&usg=AOvVaw2PEz1LVzsJqGVDHz1BHOJ-

 Read on Twitter
Read on Twitter