Exclusive:
Mystery of Bharat Biotech's Phase 1&2 Covid-19 vaccine trials:
Bharat Biotech registered its Phase 1&2 trials for Covaxin with India's Clinical Trials Registry (CTR) - a division of ICMR - which happens to be Bharat Biotech's partner.
Here's some shocking details
Mystery of Bharat Biotech's Phase 1&2 Covid-19 vaccine trials:
Bharat Biotech registered its Phase 1&2 trials for Covaxin with India's Clinical Trials Registry (CTR) - a division of ICMR - which happens to be Bharat Biotech's partner.
Here's some shocking details

Phase 1 details by Bharat Biotech were registered on 1st July, 2020.
Remember - this was the time when ICMR issued a shocking letter saying "vaccine must be ready by Independence Day" so that Modi could earn brownie points.
Total number of volunteers: 1125
(1/8)
Remember - this was the time when ICMR issued a shocking letter saying "vaccine must be ready by Independence Day" so that Modi could earn brownie points.
Total number of volunteers: 1125
(1/8)
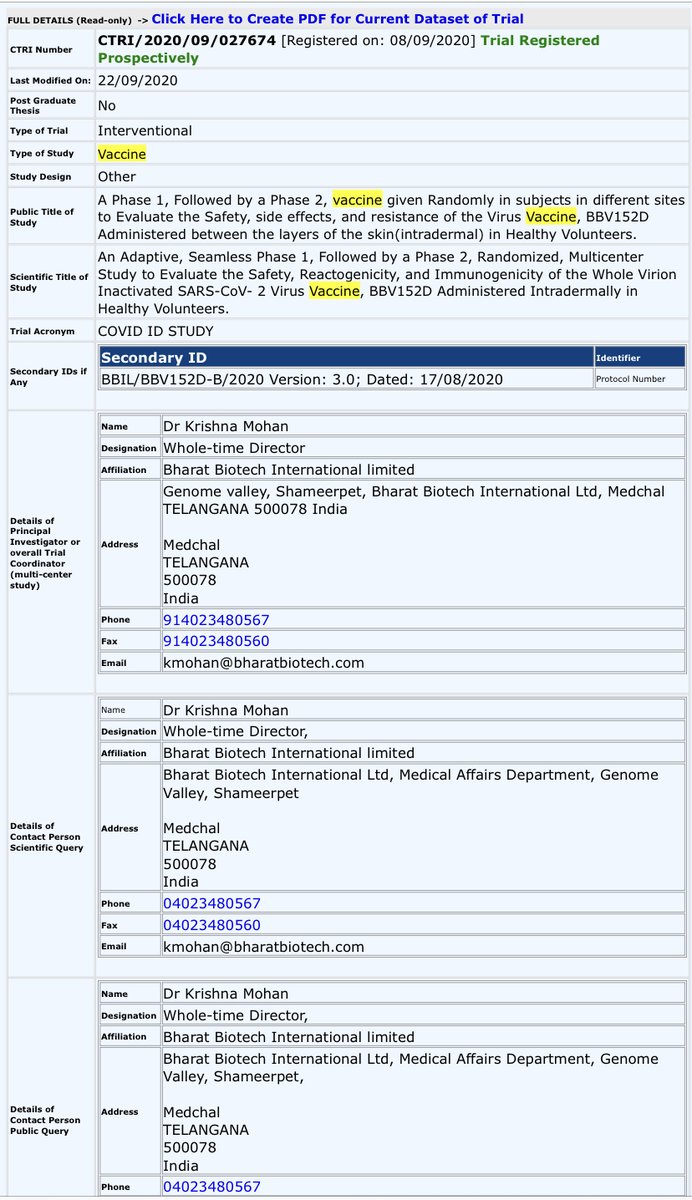
Phase 2 details by Bharat Biotech were registered on 8th September, 2020.
This was literally within 2 months of registering Phase 1 trial.
Basically, Phase 2 trial details were registered before Phase 1 was even complete.
Total number of volunteers in Phase 2: ONLY 124
(2/8)
This was literally within 2 months of registering Phase 1 trial.
Basically, Phase 2 trial details were registered before Phase 1 was even complete.
Total number of volunteers in Phase 2: ONLY 124
(2/8)
Now let's look at the duration of Phase 1 & Phase 2 trials as declared by Bharat Biotech themselves.
Phase 1 (registered on 1 Jul 2020) was to take 8 months i.e. by March 2021.
Phase 2 (registered on 8 Sep 2020) was to take 1 year & 3 months i.e. by December 2021.
(3/8)
Phase 1 (registered on 1 Jul 2020) was to take 8 months i.e. by March 2021.
Phase 2 (registered on 8 Sep 2020) was to take 1 year & 3 months i.e. by December 2021.
(3/8)
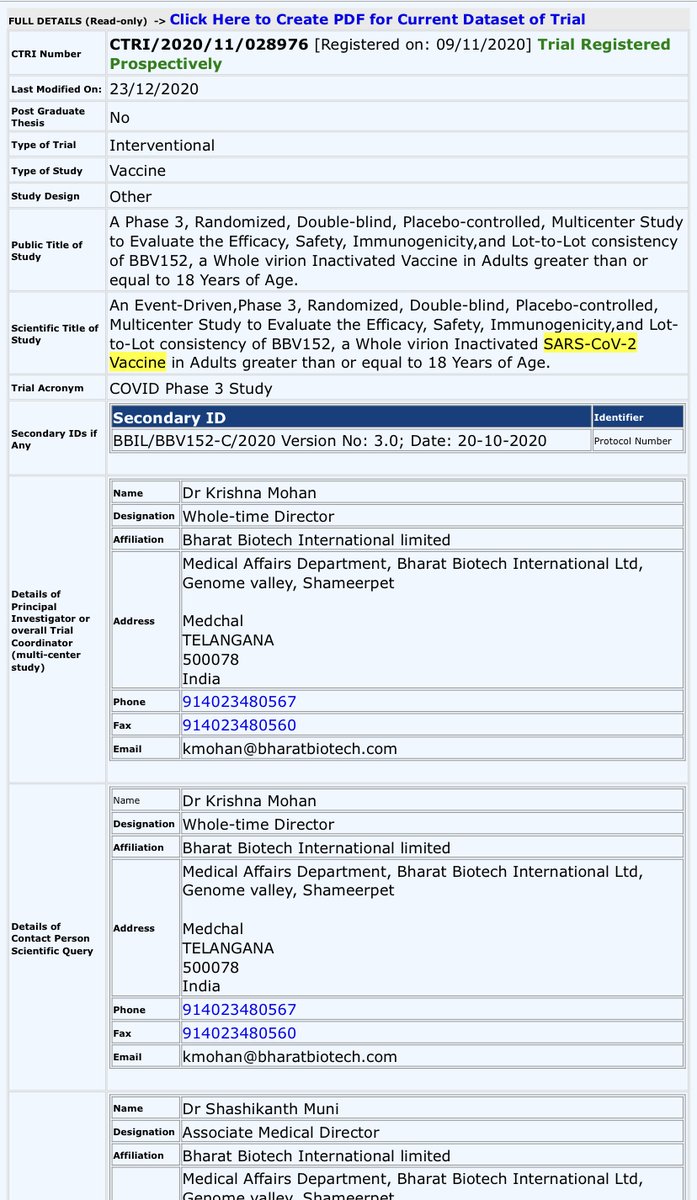
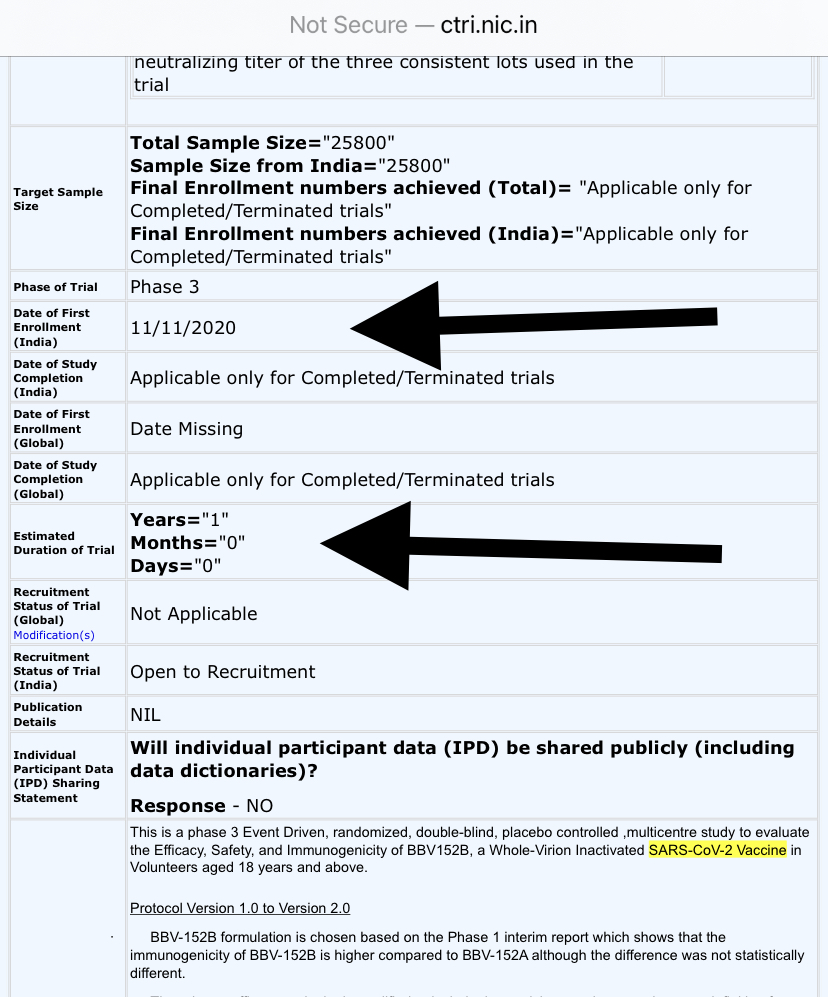
Phase 3 of Covaxin trials were registered on 9th November, 2020 with a sample size of 25,800 participants.
Registered duration for Phase 3 is 1 year i.e. by Nov 2021.
Basically, Bharat Biotech registered Phase 2 & Phase 3 trials before Phase 1 was not even completed.
(4/8)
Registered duration for Phase 3 is 1 year i.e. by Nov 2021.
Basically, Bharat Biotech registered Phase 2 & Phase 3 trials before Phase 1 was not even completed.
(4/8)
To sum it up:
Phase 1 to be completed by Mar 2021
Phase 2 to be completed by Dec 2021
Phase 3 to be completed by Nov 2021 (that's, bizarrely, 1 month BEFORE Phase 2)
And yet - Bharat Biotech's Covaxin has been approved by the Indian govt in January 2021.
(5/8)
Phase 1 to be completed by Mar 2021
Phase 2 to be completed by Dec 2021
Phase 3 to be completed by Nov 2021 (that's, bizarrely, 1 month BEFORE Phase 2)
And yet - Bharat Biotech's Covaxin has been approved by the Indian govt in January 2021.
(5/8)
On what basis was this approval given when, by Bharat Biotech's own registration documents, Phase 1 completion is still 3 months away?
Is this why the Modi govt is reluctant to share data for Bharat Biotech's Phase 1 & 2 trials publicly?
(6/8)
Is this why the Modi govt is reluctant to share data for Bharat Biotech's Phase 1 & 2 trials publicly?
(6/8)
The TOTAL number of volunteers in Bharat Biotech's Phase 1 AND 2 trials is 1249 people.
For a country with a population of 1.3 billion people.
How on earth is this vaccine "110% safe" & "works against the mutated strain"?
Where's the Phase 1 & 2 data to prove this?
(7/8)
For a country with a population of 1.3 billion people.
How on earth is this vaccine "110% safe" & "works against the mutated strain"?
Where's the Phase 1 & 2 data to prove this?
(7/8)
The Modi govt must IMMEDIATELY release the Phase 1 & Phase 2 data of Bharat Biotech's Covaxin trials to the public.
It must also explain how trials, which were supposed to take 1.5 years, got done in 5 months.
The reluctance to share any data is a MASSIVE RED FLAG.
(8/8)
It must also explain how trials, which were supposed to take 1.5 years, got done in 5 months.
The reluctance to share any data is a MASSIVE RED FLAG.
(8/8)
Data sourced from Indian Govt's "Clinical Trial Registry - India" (CTRI) which comes under the ICMR:
1. http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=45184&EncHid=&userName=bbv152
2. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=46312&EncHid=&userName=vaccine
3. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=48057&EncHid=&userName=sars-cov-2%20vaccine
1. http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=45184&EncHid=&userName=bbv152
2. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=46312&EncHid=&userName=vaccine
3. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=48057&EncHid=&userName=sars-cov-2%20vaccine

 Read on Twitter
Read on Twitter