Curious case of Covishield in India.
As we know how a “mistake” of wrong dose which otherwise vitiates the whole trial took the efficacy of Oxford AstraZeneca’s ChAdOx1-S from 60 % to 70%, its indian counterpart had got approval for “restricted emergency use” in India. (1/n)
As we know how a “mistake” of wrong dose which otherwise vitiates the whole trial took the efficacy of Oxford AstraZeneca’s ChAdOx1-S from 60 % to 70%, its indian counterpart had got approval for “restricted emergency use” in India. (1/n)
In India it is being manufactured by SII.
In order to get approval for a drug to be manufactured by a different manufacturer with same combination of ingredients (a variation 20 % is permissible in global practices) and same process, a bioavailability, bioequivalence (2/n)
In order to get approval for a drug to be manufactured by a different manufacturer with same combination of ingredients (a variation 20 % is permissible in global practices) and same process, a bioavailability, bioequivalence (2/n)
And safety study is done by comparing immunogenicity of the new drug to reference drug and helps in getting easy approval, this hel new manufacturer to let go clinical trials in case of emergency usages as the safety and efficacy of reference drug is pre-established and (3/n)
Well tested.
But, can approval (even for restricted use) be given to such a drug without clinical trials for efficacy and safety who’s reference drug itself only has emergency use authorization and its safety and efficacy is not well established. Answer would be NO. (4/n)
But, can approval (even for restricted use) be given to such a drug without clinical trials for efficacy and safety who’s reference drug itself only has emergency use authorization and its safety and efficacy is not well established. Answer would be NO. (4/n)
But, of course its a raging pandemic so exception is made. Again, the reference drug was never assessed by our drug regulator, its emergency usage approval is by a foreign regulator, moreover phase-3 trials of the reference drug were itself contaminated with some (5/n)
“astonishing mistakes”, which thus not inspire much confidence.
Ok, let it be we need that vaccine afterall its pandemic, we can have a phase-3 clinical trial in our country and assess its safety and efficacy in our own significant population this rule out issue of (6/n)
Ok, let it be we need that vaccine afterall its pandemic, we can have a phase-3 clinical trial in our country and assess its safety and efficacy in our own significant population this rule out issue of (6/n)
Ethnicity variation too. Fine enough.
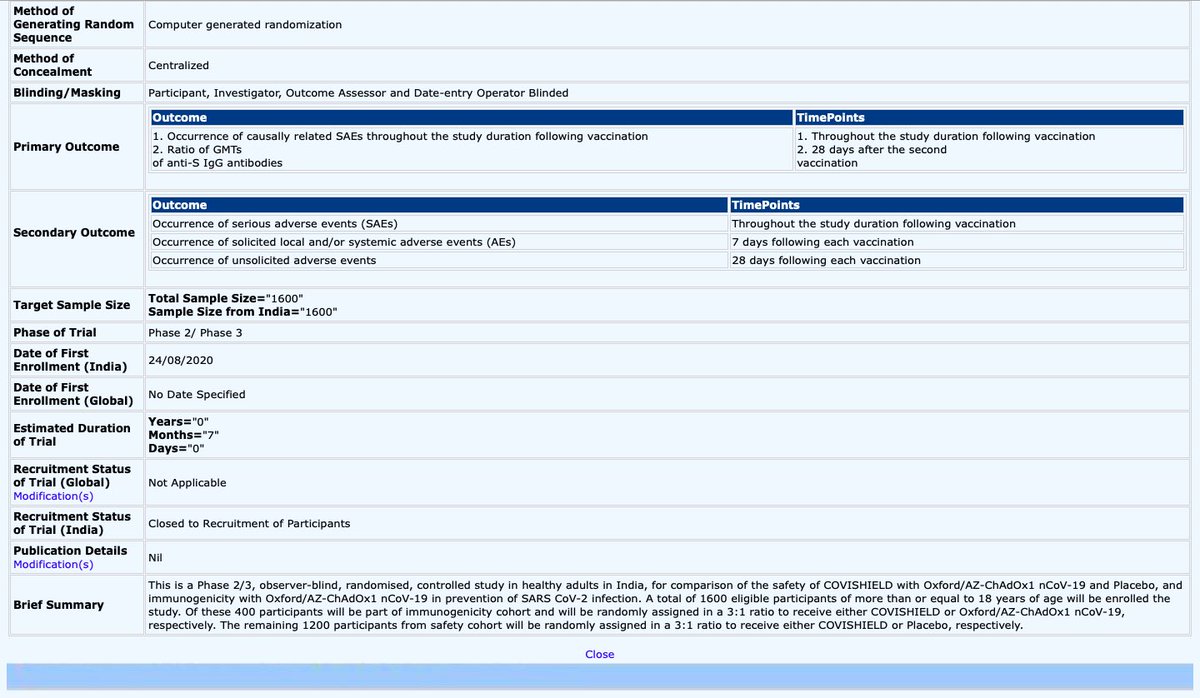
But, are trials being conducted to assess efficacy of vaccine?
And what is the sample size on which safety is being assessed?
The answer is shockingly NO, trials of Covishield in India doesn’t evaluates its efficacy, its only (7/n)
But, are trials being conducted to assess efficacy of vaccine?
And what is the sample size on which safety is being assessed?
The answer is shockingly NO, trials of Covishield in India doesn’t evaluates its efficacy, its only (7/n)
Evaluates its immunogenicity by taking a sample size of as large as 400 people where they expect to sell at least 500 million doses each costing 1000/-.
While sample size to evaluate safety is humongous 1200 people among 1.3 billion people, one can expect what might be (8/n)
While sample size to evaluate safety is humongous 1200 people among 1.3 billion people, one can expect what might be (8/n)
The wide range of different age groups, gender, different people with various pre existing conditions and so on it covers and how remarkable is going to be this evaluation.
No I am not kidding see it yourself.
Here is a link to details of trial
http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=46186&EncHid=&userName=covid-19%20vaccine
(9/n)
No I am not kidding see it yourself.
Here is a link to details of trial
http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=46186&EncHid=&userName=covid-19%20vaccine
(9/n)
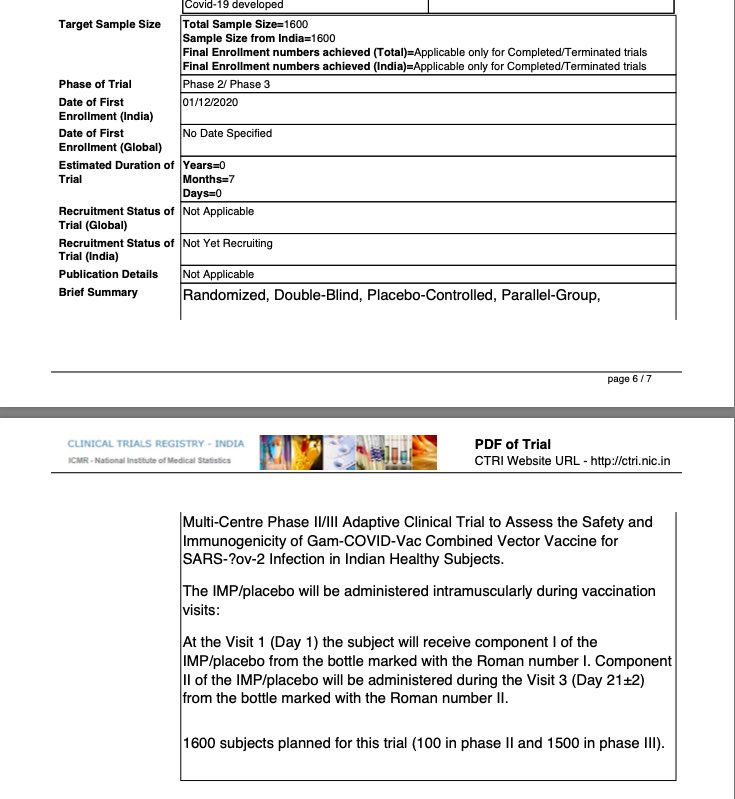
This is not alone on this another viral vector candidate Russian Sputnik V which had entered into collaboration with Dr Reddy’s is also conducting Phase 2 trial on 100 volunteers and Phase 3 trial on 1500 trials in which it doesn’t evaluates its efficacy but only (10/n)
evaluates Immunogenicity and safety of the vaccine candidate.
We all know how reliable the data from Russian agencies is believed.
We all know, its a pandemic so we might need to go for a shortcut but Double Shortcut?
In both these vaccine candidate efficacy data is (11/n)
We all know how reliable the data from Russian agencies is believed.
We all know, its a pandemic so we might need to go for a shortcut but Double Shortcut?
In both these vaccine candidate efficacy data is (11/n)
As good as non-existent.
Here is link to trial details of sputnik V in India
http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=49102&EncHid=&modid=&compid=%27,%2749102det%27
How come Indian Drug regulator is allowing this approach which seriously jeopardizes approval processes?
Why they are not evaluating efficacy in Indian trials?
(12/n)
Here is link to trial details of sputnik V in India
http://ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=49102&EncHid=&modid=&compid=%27,%2749102det%27
How come Indian Drug regulator is allowing this approach which seriously jeopardizes approval processes?
Why they are not evaluating efficacy in Indian trials?
(12/n)
While there had been much chaos (though rightly) around, regrading absence of phase 3 efficacy data of Bharat Biotech’s Covaxin and despite it getting a strange “Clinical Trial Mode Restricted use” approval, why we aren’t questions other strange approvals?
Even for (13/n)
Even for (13/n)
Sake of argument presuming efficacy data of viral vector candidate to be uncontaminated, Should a vaccine be given restricted use approval without testing efficacy in our population setting and merely on basis of similar immunogenicity, when the reference vaccine (14/n)
is itself not well tested neither have a full approval that too from a foreign regulator.
Always keep in mind,
MEDICINE IS NOT MATHEMATICS.
It is quite similar to saying that we are reading same books so we will get equal marks.
As far as legality is concerned (15/n)
Always keep in mind,
MEDICINE IS NOT MATHEMATICS.
It is quite similar to saying that we are reading same books so we will get equal marks.
As far as legality is concerned (15/n)
Schedule 2 of Clinical trial rules, 2019, sub clause (d) provide “accelerated approval" to “investigational new drugs” for the purpose of treatment (not vaccination/prevention) on the basis of phase 2 trial data incase of life threatening diseases, in absence of alternate (16/n)
Treatment but this approval is conditional and manufacturer have to conduct further phases of clinical trial and present the data for further evaluation. This doesn’t absolves them from clinical trial.
Now, the very definition of clinical trial in rules include both (17/n)
Now, the very definition of clinical trial in rules include both (17/n)
evaluation of safety and efficacy, so the further clinical trials had to provide data on both.
The approvals of both vaccines till now is legal, if we ignore the very fact that treatment doesn’t mean giving vaccine to an otherwise health person.
(18/n)
The approvals of both vaccines till now is legal, if we ignore the very fact that treatment doesn’t mean giving vaccine to an otherwise health person.
(18/n)
As far as Covaxin is concerned it had provided phase 2 clinical trial data and is conducting phase 3 clinical trial which evaluates both efficacy and safety data.
http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=48057&EncHid=&userName=CTRI/2020/11/028976
But in case of other candidates as they are not evaluating efficacy and only (19/n)
http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=48057&EncHid=&userName=CTRI/2020/11/028976
But in case of other candidates as they are not evaluating efficacy and only (19/n)
Evaluating safety and immunogenicity they are breaching the very need of doing a clinical trial.
Clinical trials are conducted to ascertain what is being claimed by manufacturer and in case of a vaccine it is its efficacy which is the claim.
So prove it.
(20/n)
Clinical trials are conducted to ascertain what is being claimed by manufacturer and in case of a vaccine it is its efficacy which is the claim.
So prove it.
(20/n)
Clinical Trial Rules, 2019 doesn’t provides for accelerated approval of drugs on the basis of mere bioequivalence study comparing immunogenicity and safety study as has been done in case of Covishield and thus not legal.
Even presuming by mending and bending regulations (21/n)
Even presuming by mending and bending regulations (21/n)
It is lawful to grant such approval on basis of bioequivalence study by comparing a novel unproven reference drug and the new drug but for that too one need to compare immunogenicity of the two and need conclusive data on it.
The ongoing study of covishield having (22/n)
The ongoing study of covishield having (22/n)
Participants is assessing the same and its results are still not out.
Then how the very conclusion that Covishield is bioequivalent was reached on the very first place?
How can an accelerated approval be given on basis of efficacy data or rather any data of a reference (23/n)
Then how the very conclusion that Covishield is bioequivalent was reached on the very first place?
How can an accelerated approval be given on basis of efficacy data or rather any data of a reference (23/n)
drug when it is not established that drug seeking approval is a bio equivalent of reference drug?
Borrowing words from recent statements- It could be water too though the data of safety study is too not available to say it is as harmless as water as its an ongoing study (24/n)
though the data of safety study is too not available to say it is as harmless as water as its an ongoing study (24/n)
Borrowing words from recent statements- It could be water too
 though the data of safety study is too not available to say it is as harmless as water as its an ongoing study (24/n)
though the data of safety study is too not available to say it is as harmless as water as its an ongoing study (24/n)
To put it straight we don’t even have enough data to even conclude that Covishield is equivalent of AstraZeneca’s ChAdOx1-S.
Ideally for comparing immunogenicity of two drugs, same assays should be used so that the variation because of use of different assays doesn’t (25/n)
Ideally for comparing immunogenicity of two drugs, same assays should be used so that the variation because of use of different assays doesn’t (25/n)
Occur and data of two drugs are comparable.
Whether it is being followed in this case? We really don’t know anything about it.
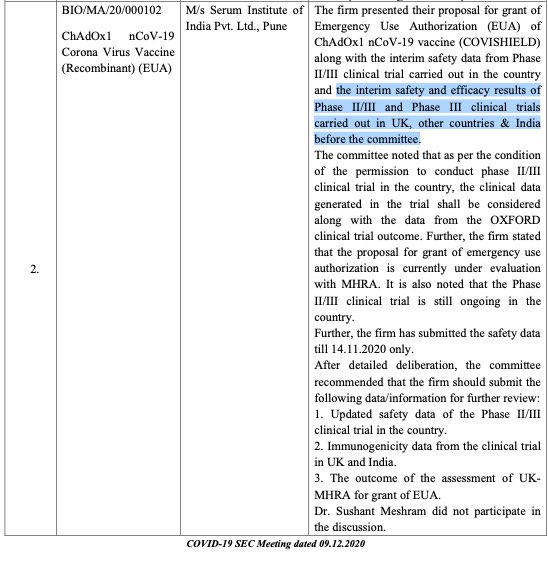
See SEC recommendations on covishield on 09/12/2021,
https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/common_download.jsp?num_id_pk=MTI3NQ==
it says SII had submitted interim safety and efficacy results (26/n)
Whether it is being followed in this case? We really don’t know anything about it.
See SEC recommendations on covishield on 09/12/2021,
https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/common_download.jsp?num_id_pk=MTI3NQ==
it says SII had submitted interim safety and efficacy results (26/n)
of Phase- 2 & 3 clinical trials conducted in UK, other in UK, other countries & India before the committee of covishield. But trial registration details with Clinical Trial Registry of India CTRI
http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=46186&EncHid=&userName=covid-19%20vaccine
says the trial is not assessing efficacy and (27/n)
http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=46186&EncHid=&userName=covid-19%20vaccine
says the trial is not assessing efficacy and (27/n)
Rather only evaluating safety and immunogenicity of the drug.
Then from where and how on earth on 09/12/2021 SEC went through interim data on efficacy submitted by SII from phase 2 and 3 trial in India.
SEC recommendations of 01/01/2021 says safety and immunogenicity (28/n)
Then from where and how on earth on 09/12/2021 SEC went through interim data on efficacy submitted by SII from phase 2 and 3 trial in India.
SEC recommendations of 01/01/2021 says safety and immunogenicity (28/n)
data of India is comparable with the same of overseas.
https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/common_download.jsp?num_id_pk=MTMwMQ==
But, Where is the data? Have anyone else than SEC had a look at it?
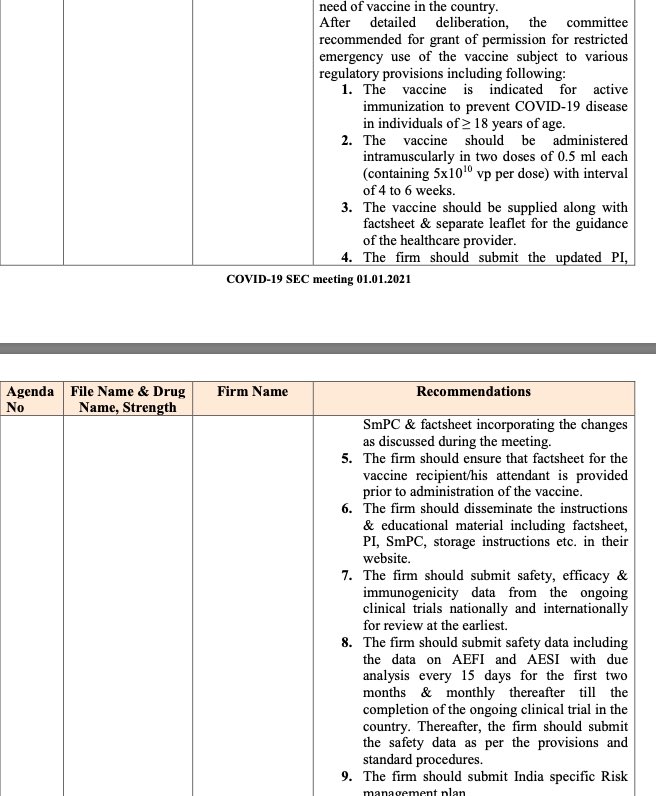
See condition no. 07 upon which permission of restricted emergency use had been given by which it is required to submit (29/n)
https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/common_download.jsp?num_id_pk=MTMwMQ==
But, Where is the data? Have anyone else than SEC had a look at it?
See condition no. 07 upon which permission of restricted emergency use had been given by which it is required to submit (29/n)
safety, efficacy & immunogenicity data from the ongoing national clinical trials, but when there is evaluation of efficacy taking place from where this data on efficacy will come?
Often a bridging study by many is misconstrued as a mere bioequivalence which is wrong (30/n)
Often a bridging study by many is misconstrued as a mere bioequivalence which is wrong (30/n)
A bridging study does include evaluation of efficacy too though regulators can give relaxations on sample size.
This is a classic case of a bridge not connecting two piece of land rather two black holes with two many unknowns.
Unfortunately, this not only covishield but (31/n)
This is a classic case of a bridge not connecting two piece of land rather two black holes with two many unknowns.
Unfortunately, this not only covishield but (31/n)
This strange dubious way is used by many other vaccine candidates too, which seriously jeopardizes not only public health of India but also of other poorer nations who would rely on indian approvals of vaccines as vaccines are being manufactured in India.
When the pharma (32/n)
When the pharma (32/n)
Companies are sitting with huge orders as high as 200 million doses, is it a proper phase-3 clinical trial is too much to ask for?
Or lives of citizens of poorer nations is too inexpensive?
While European and American companies are meeting out vaccine needs of first (33/n)
Or lives of citizens of poorer nations is too inexpensive?
While European and American companies are meeting out vaccine needs of first (33/n)
First world countries, Indian companies will be meeting out vaccine needs of third world countries.
While we very rightly question BB’s Covaxin and expects phase-3 data though the law doesn’t mandates it and apply the British MHRA and American FDA standards here (34/n)
While we very rightly question BB’s Covaxin and expects phase-3 data though the law doesn’t mandates it and apply the British MHRA and American FDA standards here (34/n)
Shouldn’t we be asking the same for Covishield?
To put in proper prospective though we will know the efficacy of BB’s Covaxin sooner or later as trial for evaluating it is going on, but we won’t ever be knowing knowing what is the efficacy of Covishield. And it is so. (35/n)
To put in proper prospective though we will know the efficacy of BB’s Covaxin sooner or later as trial for evaluating it is going on, but we won’t ever be knowing knowing what is the efficacy of Covishield. And it is so. (35/n)

 Read on Twitter
Read on Twitter