The expert committee of CDSCO met on 30/12, 01/01 and 02/01 to deliberate on accelerated approval of vaccines. Recommendations made on each day have been officially published.
 In this thread, we take a look at the timeline of events that lead to the approval. Must read.
In this thread, we take a look at the timeline of events that lead to the approval. Must read.
1/n
 In this thread, we take a look at the timeline of events that lead to the approval. Must read.
In this thread, we take a look at the timeline of events that lead to the approval. Must read.1/n
30th December:
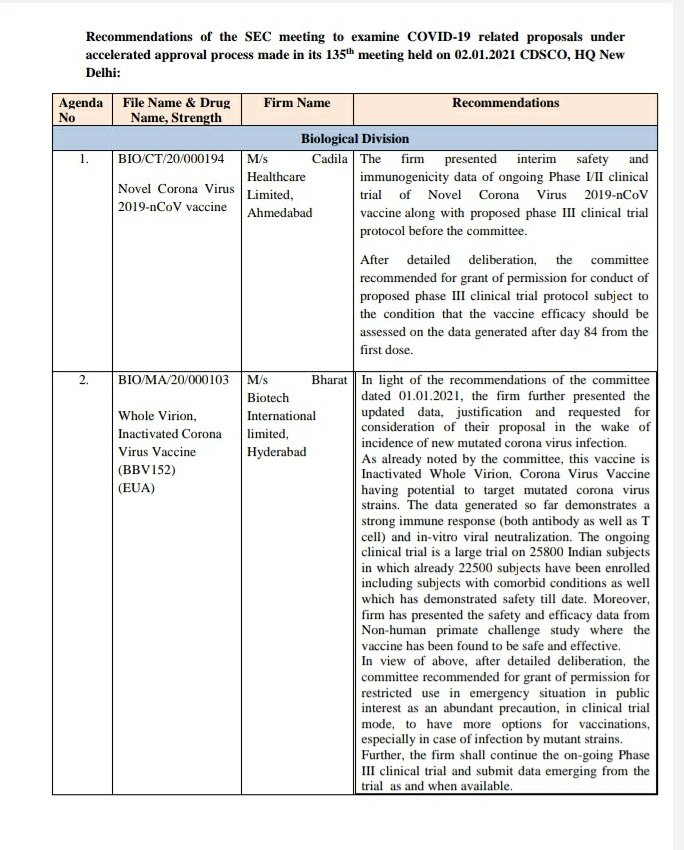
#Covaxin: Bharat Biotech presents status of Phase 3 trials and updated safety data.
 Committee recommends that updated safety and EFFICACY data of Phase 3 trials have to be presented for further consideration.
Committee recommends that updated safety and EFFICACY data of Phase 3 trials have to be presented for further consideration.
2/n
#Covaxin: Bharat Biotech presents status of Phase 3 trials and updated safety data.
 Committee recommends that updated safety and EFFICACY data of Phase 3 trials have to be presented for further consideration.
Committee recommends that updated safety and EFFICACY data of Phase 3 trials have to be presented for further consideration.2/n
30th December:
#Covishield: SII presents safety and efficacy data of Phase 2/3 trials from UK, Brazil, SA along with data from ongoing Indian trials. SII mentions that UK has provided EUA.
 Committee asks for complete details of UK approval to be submitted.
Committee asks for complete details of UK approval to be submitted.
3/n
#Covishield: SII presents safety and efficacy data of Phase 2/3 trials from UK, Brazil, SA along with data from ongoing Indian trials. SII mentions that UK has provided EUA.
 Committee asks for complete details of UK approval to be submitted.
Committee asks for complete details of UK approval to be submitted.3/n
1st January:
#Covaxin: Bharat Biotech presents data from Phase 1,2 trials as well as ongoing Phase 3. Efficacy yet to be demonstrated.
 Committee asks for Phase 3 recruitment to be expedited and present Phase 3 efficacy data for consideration for emergency use approval.
Committee asks for Phase 3 recruitment to be expedited and present Phase 3 efficacy data for consideration for emergency use approval.
4/n
#Covaxin: Bharat Biotech presents data from Phase 1,2 trials as well as ongoing Phase 3. Efficacy yet to be demonstrated.
 Committee asks for Phase 3 recruitment to be expedited and present Phase 3 efficacy data for consideration for emergency use approval.
Committee asks for Phase 3 recruitment to be expedited and present Phase 3 efficacy data for consideration for emergency use approval.4/n
1st January:
#Covishield: SII presents details of UK approval.
 Committee reviews data and notes that the safety/immunogenicity data of India trials is comparable to overseas trials.
Committee reviews data and notes that the safety/immunogenicity data of India trials is comparable to overseas trials.
Decides to recommend for restricted emergency use.
5/n
#Covishield: SII presents details of UK approval.
 Committee reviews data and notes that the safety/immunogenicity data of India trials is comparable to overseas trials.
Committee reviews data and notes that the safety/immunogenicity data of India trials is comparable to overseas trials. Decides to recommend for restricted emergency use.
5/n
Conditions of #Covishield restricted approval:
 Approved for >= 18 years of age.
Approved for >= 18 years of age.
 2 doses, 4 to 6 weeks apart.
2 doses, 4 to 6 weeks apart.
 SII should submit details of of ongoing Phase 3 studies.
SII should submit details of of ongoing Phase 3 studies.
 Safety data to be submitted every 15 days for first 2 months.
Safety data to be submitted every 15 days for first 2 months.
 Submit risk management plan.
Submit risk management plan.
6/n
 Approved for >= 18 years of age.
Approved for >= 18 years of age. 2 doses, 4 to 6 weeks apart.
2 doses, 4 to 6 weeks apart. SII should submit details of of ongoing Phase 3 studies.
SII should submit details of of ongoing Phase 3 studies. Safety data to be submitted every 15 days for first 2 months.
Safety data to be submitted every 15 days for first 2 months. Submit risk management plan.
Submit risk management plan.6/n
2nd January:
#Covaxin: Bharat Biotech presents data. Requests for approval in light of new mutated strain.
 Committee recommends for emergency use in clinical trial mode as a precaution, to have more vaccine options in light of mutant strains.
Committee recommends for emergency use in clinical trial mode as a precaution, to have more vaccine options in light of mutant strains.
Phase 3 to continue.
7/n
#Covaxin: Bharat Biotech presents data. Requests for approval in light of new mutated strain.
 Committee recommends for emergency use in clinical trial mode as a precaution, to have more vaccine options in light of mutant strains.
Committee recommends for emergency use in clinical trial mode as a precaution, to have more vaccine options in light of mutant strains. Phase 3 to continue.
7/n
In summary:
 Approval for Covishield provided on 01/01 after reviewing UK approval and trial data globally and in India.
Approval for Covishield provided on 01/01 after reviewing UK approval and trial data globally and in India.
 #Covaxin: In the meetings held on 30/12 and 01/01, SEC had asked for Phase 3 efficacy data to be presented for approval considerations.
#Covaxin: In the meetings held on 30/12 and 01/01, SEC had asked for Phase 3 efficacy data to be presented for approval considerations.
8/n
 Approval for Covishield provided on 01/01 after reviewing UK approval and trial data globally and in India.
Approval for Covishield provided on 01/01 after reviewing UK approval and trial data globally and in India. #Covaxin: In the meetings held on 30/12 and 01/01, SEC had asked for Phase 3 efficacy data to be presented for approval considerations.
#Covaxin: In the meetings held on 30/12 and 01/01, SEC had asked for Phase 3 efficacy data to be presented for approval considerations. 8/n
In the meeting on 02/01, SEC decides to recommend Covaxin for emergency approval in clinical trial mode, without efficacy data from Ph 3 trials.
Reason given being to have more vaccine options in light of mutated strains. Clarification of clinical trial mode not provided.
9/n
Reason given being to have more vaccine options in light of mutated strains. Clarification of clinical trial mode not provided.
9/n
The detailed recommendation documents of SEC by date can be found here:
https://cdsco.gov.in/opencms/opencms/en/Committees/SEC/
n/n
https://cdsco.gov.in/opencms/opencms/en/Committees/SEC/
n/n

 Read on Twitter
Read on Twitter