A (long) thread on Bharat Biotech - ICMR's #Covaxin vaccine.
1. Type of vaccine: #Covaxin is a whole virion INACTIVATED vaccine.
In such vaccines, the whole #sarscov2 virus is inactivated to make it harmless and used to make the vaccine. Seed virus provided by NIV Pune.
1/n
1. Type of vaccine: #Covaxin is a whole virion INACTIVATED vaccine.
In such vaccines, the whole #sarscov2 virus is inactivated to make it harmless and used to make the vaccine. Seed virus provided by NIV Pune.
1/n
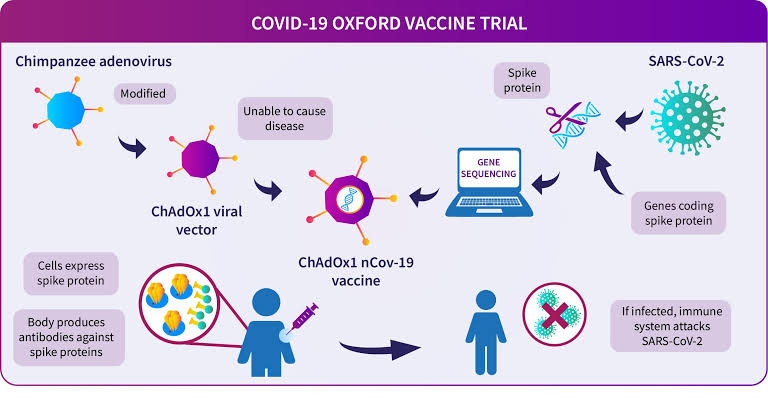
2. How is it different from #Covishield? Covishield is a VIRAL VECTOR vaccine. Here, the spike protein of #sarscov2 is sequenced. A weakened virus that causes common cold in chimpanzees called Adenovirus is used as the vector to carry the spike protein in the vaccine.
2/n
2/n
3. Phase 1 trial: 375 persons in trial, done across 11 sites in 9 states.
Age profile: 18 to 55.
375 persons divided into 4 groups. 3 groups of 100 each received 3 different formulations of the vaccine and 1 control group of 75 received placebo.
Dosage: 2 doses, 14 days.
3/n
Age profile: 18 to 55.
375 persons divided into 4 groups. 3 groups of 100 each received 3 different formulations of the vaccine and 1 control group of 75 received placebo.
Dosage: 2 doses, 14 days.
3/n
4. Phase 1 objectives: Safety and immunogenicity.
The adverse event incidents from Phase 1 given in image below. One participant in vaccine arm, tested positive and required hospitalisation. But, causally not related to vaccination.
Most adverse events reported were mild.
4/n
The adverse event incidents from Phase 1 given in image below. One participant in vaccine arm, tested positive and required hospitalisation. But, causally not related to vaccination.
Most adverse events reported were mild.
4/n
8% of placebo group showed antibodies for Covid19 in Phase 1. There was only 1 reported case of Covid19 in vaccine arm between day 0 and 75.
Satisfactory immunogenicity/presence of various antibodies was observed.
Efficacy and further safety to be assessed in Phase 3.
5/n
Satisfactory immunogenicity/presence of various antibodies was observed.
Efficacy and further safety to be assessed in Phase 3.
5/n
5. Phase 2 trial: 380 participants in Phase 2, across 9 sites (refer attached list).
Divided into 2 groups of 190 each. 2 formulations administered, no placebo.
Age profile: 12 to 65 (refer attached).
Dosage: 2 doses of 0.5mL/dose across 28 days.
6/n
Divided into 2 groups of 190 each. 2 formulations administered, no placebo.
Age profile: 12 to 65 (refer attached).
Dosage: 2 doses of 0.5mL/dose across 28 days.
6/n
No symptomatic Covid19 infections were reported in Ph 2 b/w days 0 and 75. No serious adverse event was reported until day 56. Most common events - pain, headache, fatigue, fever.
Phase 1 participants after 3 months of 2nd dose did not report any serious adverse event.
7/n
Phase 1 participants after 3 months of 2nd dose did not report any serious adverse event.
7/n
Immune response in Phase 2 with a 28 day gap between doses were higher than Phase 1 with a 14 day schedule. The study hypothesizes cell mediated antibody responses to persist atleast 6 to 12 months after 2nd dose.
 Vaccine efficacy assessments have not been carried out.
Vaccine efficacy assessments have not been carried out.
8/n
 Vaccine efficacy assessments have not been carried out.
Vaccine efficacy assessments have not been carried out. 8/n
At the end of Phase 2, the 6 micro gram formulation with Algel-IMDG as adjuvant has been selected for Phase 3 trials. 2 dose, 28 days apart.
 Efficacy, ability to limit severe cases, coverage of wider participants in age groups etc. are to be assessed in Phase 3 trial.
Efficacy, ability to limit severe cases, coverage of wider participants in age groups etc. are to be assessed in Phase 3 trial.
9/n
 Efficacy, ability to limit severe cases, coverage of wider participants in age groups etc. are to be assessed in Phase 3 trial.
Efficacy, ability to limit severe cases, coverage of wider participants in age groups etc. are to be assessed in Phase 3 trial.9/n
The Phase 3 trials, dubbed as the largest done in India expects to recruit 26,000 volunteers and started off in Nov. 2020. On 2nd Jan, 2021 - Bharat Biotech announced the recruitment of 23k participants for Phase 3 and the trial is ongoing.
10/n
10/n
Phase 3 outcomes as per CTRI:
 Primary: Evaluate efficacy to prevent symptomatic Covid19 and severe symptomatic Covid19.
Primary: Evaluate efficacy to prevent symptomatic Covid19 and severe symptomatic Covid19.
 Secondary: Evaluate efficacy to prevent severe cases, severity by age, asymptomatic cases, deaths etc. Refer attached for complete list.
Secondary: Evaluate efficacy to prevent severe cases, severity by age, asymptomatic cases, deaths etc. Refer attached for complete list.
11/n
 Primary: Evaluate efficacy to prevent symptomatic Covid19 and severe symptomatic Covid19.
Primary: Evaluate efficacy to prevent symptomatic Covid19 and severe symptomatic Covid19. Secondary: Evaluate efficacy to prevent severe cases, severity by age, asymptomatic cases, deaths etc. Refer attached for complete list.
Secondary: Evaluate efficacy to prevent severe cases, severity by age, asymptomatic cases, deaths etc. Refer attached for complete list.11/n
In summary:
 Covaxin is a whole inactivated virus vaccine, given as 2 doses over 28 days.
Covaxin is a whole inactivated virus vaccine, given as 2 doses over 28 days.
 Phase 1 and 2 trials shows safety and antibody response generation profiles.
Phase 1 and 2 trials shows safety and antibody response generation profiles.
 Phase 3 (ongoing) will primarily evaluate efficacy to prevent cases, severe cases, deaths etc.
Phase 3 (ongoing) will primarily evaluate efficacy to prevent cases, severe cases, deaths etc.
n/n
 Covaxin is a whole inactivated virus vaccine, given as 2 doses over 28 days.
Covaxin is a whole inactivated virus vaccine, given as 2 doses over 28 days. Phase 1 and 2 trials shows safety and antibody response generation profiles.
Phase 1 and 2 trials shows safety and antibody response generation profiles. Phase 3 (ongoing) will primarily evaluate efficacy to prevent cases, severe cases, deaths etc.
Phase 3 (ongoing) will primarily evaluate efficacy to prevent cases, severe cases, deaths etc.n/n

 Read on Twitter
Read on Twitter