I've been seeing a lot of discussion around the dosage gaps recommended by government for the Astra/Oxford & Pfizer/BioNTech vaccines. My thoughts on the potential benefits & risks of such an approach, and the need for much greater transparency around these decisions. Thread.
The UK govt announced recently that both the Oxford & BioNTech vaccines would be now administered with a gap of between 4-12 weeks (so prioritise administration of first dose, given limited resources).
What is the basis of this?
What is the basis of this?
The basis that's been discussed seems to be that
1) the first dose is likely to confer some degree of protection against disease, so better to roll this out as fast as possible, and
2) that for Oxford/Astra efficacy may be higher when the gap between doses is greater.
1) the first dose is likely to confer some degree of protection against disease, so better to roll this out as fast as possible, and
2) that for Oxford/Astra efficacy may be higher when the gap between doses is greater.
I discuss some of the technical details around the Oxford trial below for those interested in these. If you'd just like a summary of this, please skip to the summary tweet titled 'SUMMARY'.
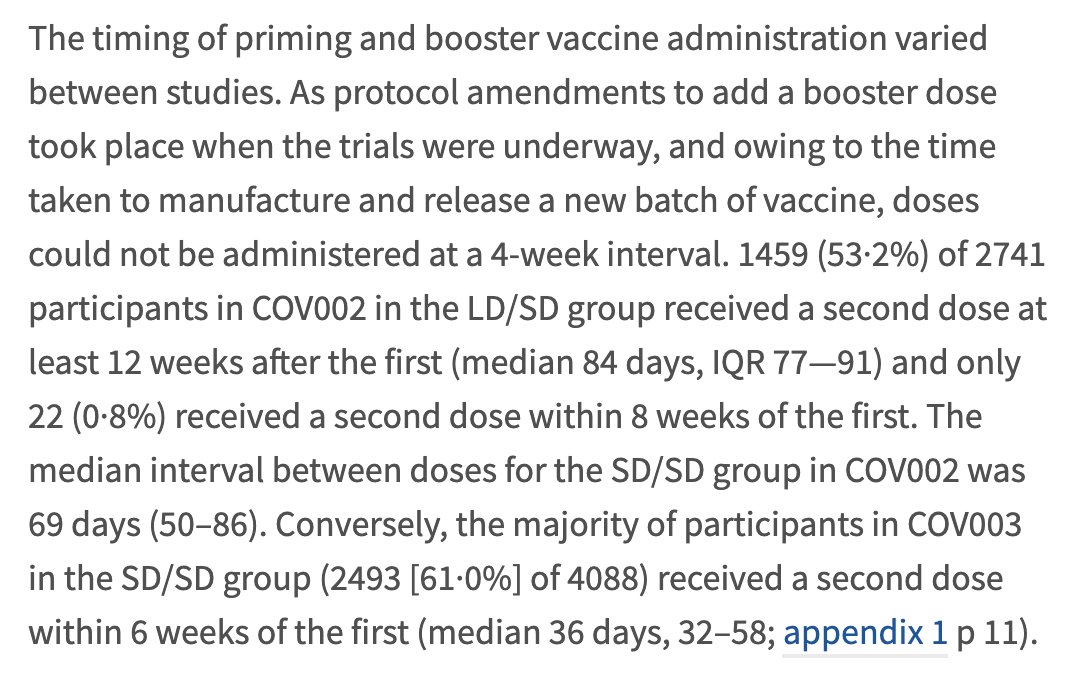
What does published evidence show? The Astra/Oxford data are a bit messy because of protocol changes during the trial. This is because the trial was originally planned as a single dose trial, but a booster dose was added when developers realised that this would likely help.
This meant the 2nd dose was added months after the start of the trial. Because of the nature of the trial- younger people recruited into the trial first & older people recruited once it was decided that 2 doses should be used, younger participants were given the 2nd dose later.
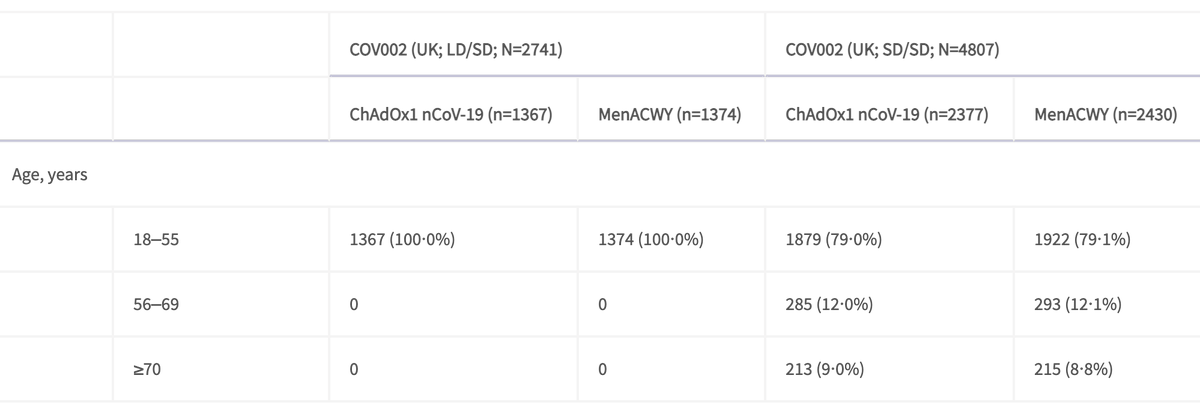
To complicate matters there was an error early in the trial which meant that ~1,400 participants were given a low 1st dose by mistake. Given the first tranche of participants were <55 years, the low dose (1st dose)/standard dose (2nd dose) combination was only used in this group
So approximately 42% of participants <55 yrs had the lowdose/standard dose (LD/SD) combination, and all the older ones had the SD/SD combination. And the gap between doses was higher among <55 yr olds vs >55 yr olds, although I couldn't find the breakdown of this in the paper.
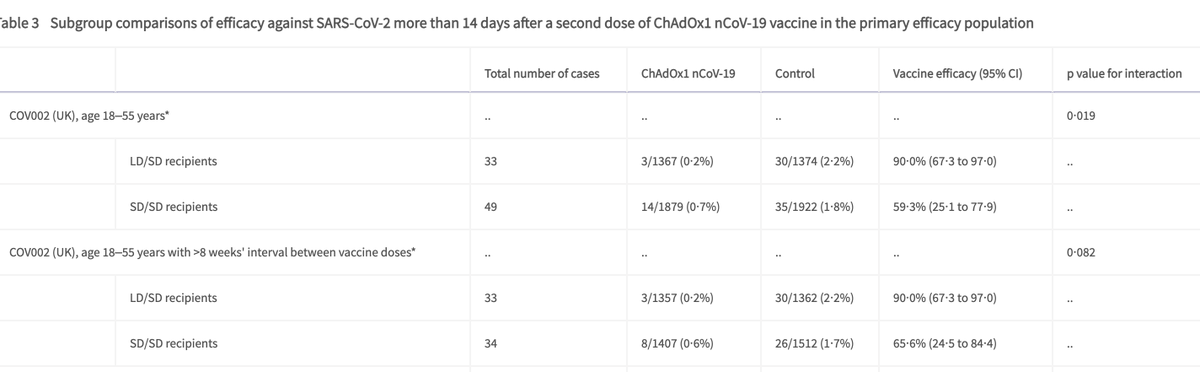
The efficacy results that are reported suggest an efficacy of ~60% in reducing disease for the SD/SD group, and ~90% for the LD/SD group. What is less clear is whether these differences are due to the lower first dose, younger participants, or longer gaps between doses.
So let's look at efficacy by age group and dose.
Vaccine efficacy among 18-55 yr olds SD/SD dosing was 59% vs LD/SD dosing at 90%.
Is this due to dosing, or differences in gaps between doses?
Differences in gaps don't appear to impact efficacy in this analysis.
Vaccine efficacy among 18-55 yr olds SD/SD dosing was 59% vs LD/SD dosing at 90%.
Is this due to dosing, or differences in gaps between doses?
Differences in gaps don't appear to impact efficacy in this analysis.
Of course we can't evaluate this in ages >55 as they only had a short gap in dosing. So we don't have any data on longer gaps in >55 yr olds, at least in the data that are public.
I have seen reports that unpublished data support longer gaps between doses. But without seeing the data, I can't evaluate the basis for this. Presumably MHRA has evaluated the evidence before granting approval for the current dosage protocol. https://www.bbc.co.uk/news/health-55280671
What about the efficacy after the first dose?
It looks like the first dose prevented all severe disease (although numbers were small) in the SARS-CoV-2 vaccine group compared to vaccine control after the first 21 days of vaccination, and <14 days after the 2nd dose.
It looks like the first dose prevented all severe disease (although numbers were small) in the SARS-CoV-2 vaccine group compared to vaccine control after the first 21 days of vaccination, and <14 days after the 2nd dose.
SUMMARY: the Oxford/Astra trial examined dosing with gaps between 4-12 wks- although longer gaps appear to be limited mostly to younger participants. There was no difference reported in published data between these & efficacy from the 1st dose seems high for severe disease.
Given this, I can understand the plan for longer dosing gaps for this vaccine, although I do think that it's important that the unpublished data that support this are released for transparency & to build public trust which is essential at this point.
The application of the same principle to the Pfizer/BioNtech vaccine is more difficult to evaluate. This vaccine was studied with a strict protocol of 21 days between doses & the company has since issued a warning that they cannot ensure efficacy if protocol is changed
While the Pfizer trial shows reasonably high efficacy after the 1st dose of vaccine (~80%), the duration of this following 21 days is unclear. So there is a risk that this level may decline after this period, as we don't have direct data on this from trials.
However, there are also arguments for the approach the govt is taking, as outlined below. Essentially the thinking is that given the urgency, roll-out across double the numbers with 80% efficacy is better than rolling out half numbers with 95% efficacy. https://twitter.com/Bob_Wachter/status/1344667655324585986?s=20
The assumptions key to this argument are of course that:
1. Resources for roll-out are limited & fixed & we need to optimise how best to use them within limitations
2. There isn't significant decline in immunity after the 3 wk mark
3. Later dosing will not affect overall efficacy
1. Resources for roll-out are limited & fixed & we need to optimise how best to use them within limitations
2. There isn't significant decline in immunity after the 3 wk mark
3. Later dosing will not affect overall efficacy
I'm not a vaccinology expert so can't really comment on 2. and 3. From reading views from other experts, the general view seems to be that later dosing is unlikely to impact overall efficacy. What level of immunity lasts up to 12 weeks is less clear, I think.
I'm also not entirely clear about the impact of a longer period of partial immunity (if immunity declines following 3 wks) on viral adaptation. I know some experts have raised concerns about this. I think this is dependent on how much immunity declines prior to the 2nd dose.
Given a lot of this is conjecture, it's hard to know whether this is the right strategy for the Pfizer vaccine. I am naturally cautious about changing a protocol that's tried & tested to one where we don't have data on, particularly given the warnings issued by Pfizer.
I'm also concerned by the poor planning around this. Given these data were available for a while, it's not clear to me why this decision was made now, when hundreds of thousands have had their second appts booked in. Cancelling these is also time-consuming & undermines trust.
Irrespective of how and why the MHRA approved this, I think it's important to release the thinking & the data around it - to create transparency & build trust with the public, and with health-care workers. It's very clear that HCWs were not consulted in this, which led to chaos.
Such actions really risk undermining public trust, when it's already very low. We are counting on high vaccine uptake to be able to contain the pandemic - we can't afford to not take the public along on decisions such as this. Good communication & transparency is key.
Given the urgency of vaccine roll-out now, these are complex decisions. While there may be good scientific reasons for which these decisions have been made, it's important that these reasons are made clear & the public and HCWs are engaged in this decision-making process.
Just wanted to highlight one last point- it looks like the govt have released a document around this today.
A key part of the rationale appears to be a bottleneck in vaccine supply (rather than roll-out). https://twitter.com/portefeuillefun/status/1344729532826349572?s=20
A key part of the rationale appears to be a bottleneck in vaccine supply (rather than roll-out). https://twitter.com/portefeuillefun/status/1344729532826349572?s=20

 Read on Twitter
Read on Twitter