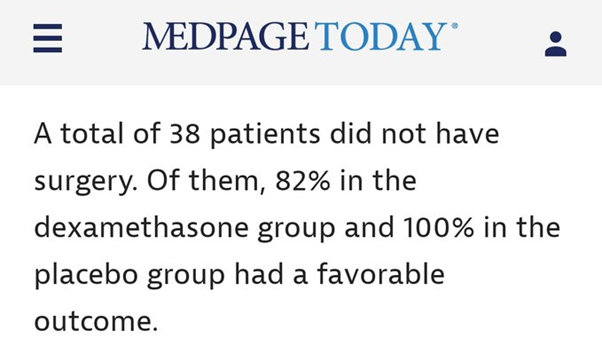A thread on the recently published #Dex-CSDH trial – aiming to cover many of the common questions that have been asked in the past few days. https://www.nejm.org/doi/10.1056/NEJMoa2020473
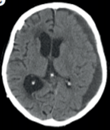
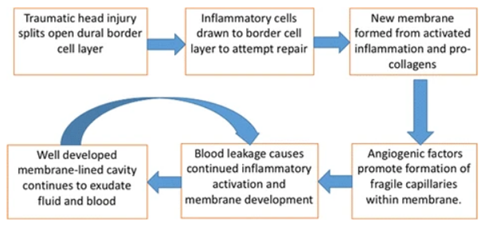
Chronic subdural haematoma ( #CSDH) is a common neurological condition in elderly individuals, and its incidence is rising due to an ageing population and increasing use of anticoagulant and antiplatelet medication.
#Neurosurgery #neurology #geriatrics
#Neurosurgery #neurology #geriatrics
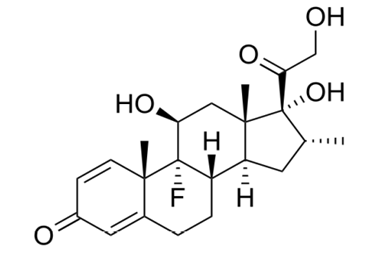
Dexamethasone is a potent synthetic member of the glucocorticoid class of steroid drugs that has anti-inflammatory and immunosuppressant effects.
It is used for a range of neurological conditions.
It is used for a range of neurological conditions.
Inflammation plays a central role in the pathogenesis of #CSDH. A good recent review by @EdlmannE can be found here https://pubmed.ncbi.nlm.nih.gov/28558815/ (open access)
Surgery is the mainstay of management of symptomatic patients – and has good results (recent UK data https://pubmed.ncbi.nlm.nih.gov/27834599/ ).
Dexamethasone, due to its anti-inflammatory effects, has been used as an adjunct or alternative to surgery since the 1970s.
Dexamethasone, due to its anti-inflammatory effects, has been used as an adjunct or alternative to surgery since the 1970s.
A couple of good systematic reviews had contradictory conclusions with regards to its safety profile and clinical efficacy ( https://pubmed.ncbi.nlm.nih.gov/24096761/ and https://pubmed.ncbi.nlm.nih.gov/22642223/ ).
Hence, we sought to provide high-quality evidence by conducting a multi-centre, placebo-controlled RCT.
The protocol was written collaboratively by neurosurgeons & trainees from several UK units (including
@pja_hutch @hani_marcus @aswinchari @EdNeuroSurg @EdlmannE)
The protocol was written collaboratively by neurosurgeons & trainees from several UK units (including
@pja_hutch @hani_marcus @aswinchari @EdNeuroSurg @EdlmannE)
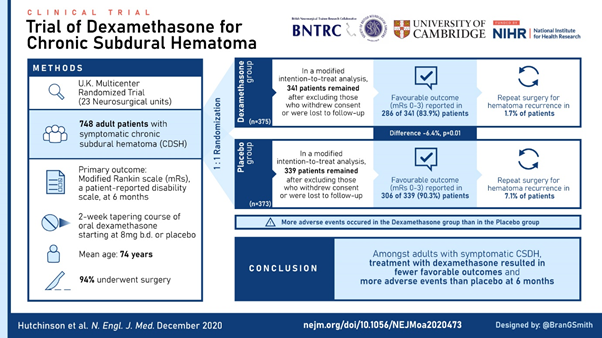
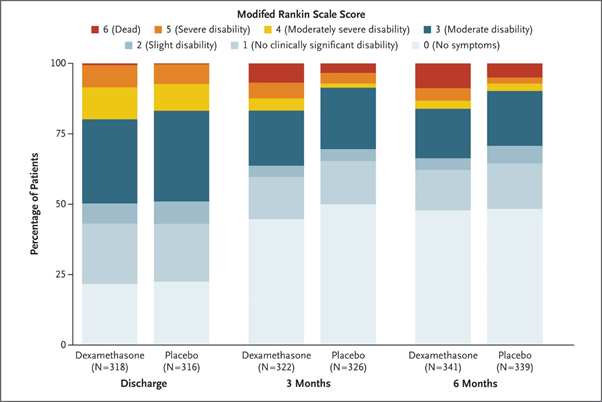
The trial recruited 748 patients in just over 3 years and was published last week online @NEJM. The visual abstract (thanks to @BranGSmith) is below.
The results are interesting as although dexamethasone reduced post-op recurrence, it led to more unfavourable outcomes at 6 months (primary outcome measure). Hence, on this basis, we have recommended that the use of dexamethasone for #CSDH should STOP 

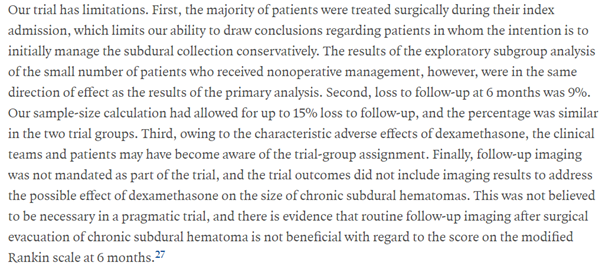
Every study has limitations and Dex-CSDH is not an exception.
The first one mentioned below is an important one and hopefully the ongoing Dutch DECSA trial will provide further evidence ( https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-018-2945-4)
The first one mentioned below is an important one and hopefully the ongoing Dutch DECSA trial will provide further evidence ( https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-018-2945-4)
However, the subgroup analysis of non-operated patients (n.b. small numbers) showed a similar direction of effect as the primary analysis.
So, I will stop using them altogether for #CSDH - and revisit this after the DECSA trial reports.
So, I will stop using them altogether for #CSDH - and revisit this after the DECSA trial reports.
The support of all co-applicants, PIs/co-PIs, collaborators, research nurses in all 23 sites was amazing – many thanks to everyone. Finally, we sincerely thank all patients & carers, as without their altruistic participation, the #DexCSDH trial would not have been possible.

 Read on Twitter
Read on Twitter