Excited to share that our paper is out today in @NatureComms https://www.nature.com/articles/s41467-020-19917-0. A great collaboration from @DKirschMDPhD @YvonneMowery @DukeRadOnc @DukeCancer and @Barzin_
@max_diehn @StanfordCancer examining mechanisms of primary tumor resistance to RT and PD-1 blockade.
@max_diehn @StanfordCancer examining mechanisms of primary tumor resistance to RT and PD-1 blockade.
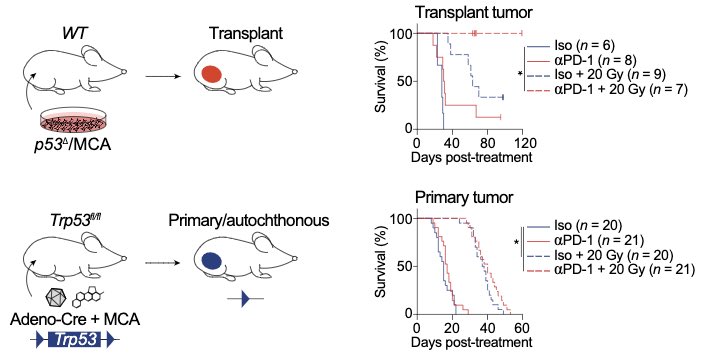
We set out to understand why PD-1 blockade and radiotherapy are so much more effective (sometimes 100% effective!) in mouse models of cancer than they are in most patients. We used a model of sarcoma with high TMB, examining tumor and immune cells in the tumor microenvironment.
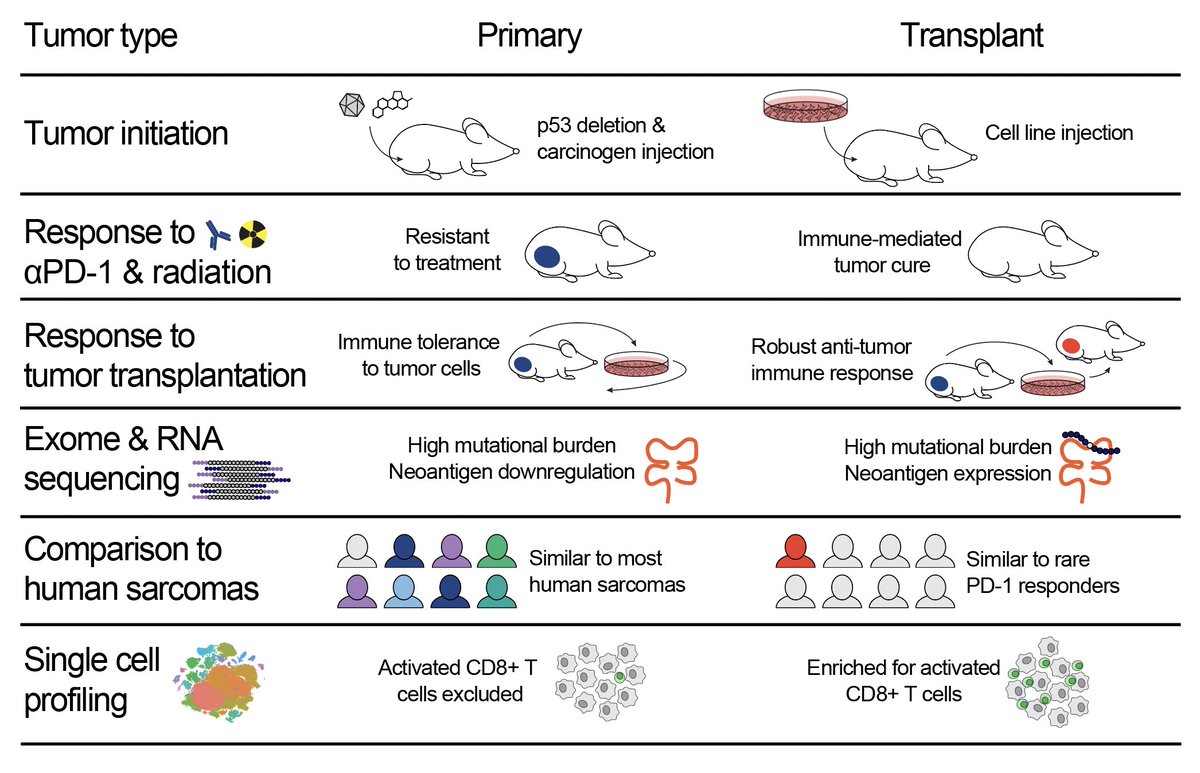
Most preclinical immunotherapy studies use transplant tumor models. We found that transplant sarcomas are cured by PD-1 blockade and radiotherapy, but the same treatment fails to cure the identical tumor model in the absence of transplantation (i.e. in primary tumors).
We found that making a tumor cell line leads to upregulation of tumor neoantigen expression, which is maintained when the cells are injected to generate transplant tumors. In primary tumors, the immune system decreases the number & expression of neoantigens during tumor formation
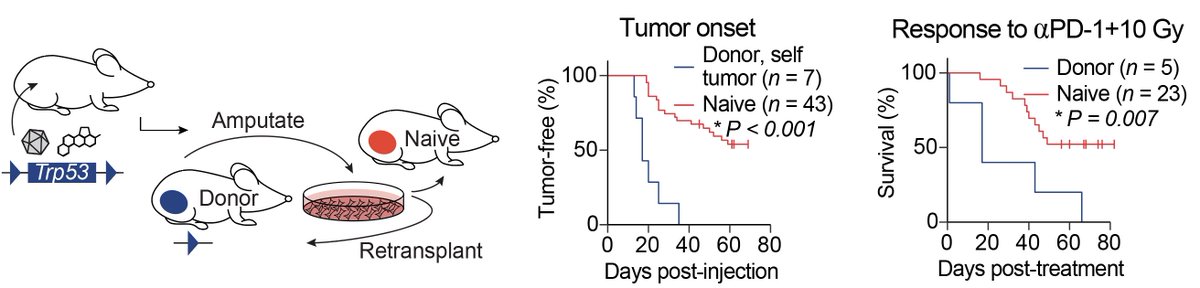
In addition to studying tumor cells, we also looked at how these tumor models change the immune system. Using auto-transplantation experiments, we discovered tumor-specific immune tolerance in the primary model system.
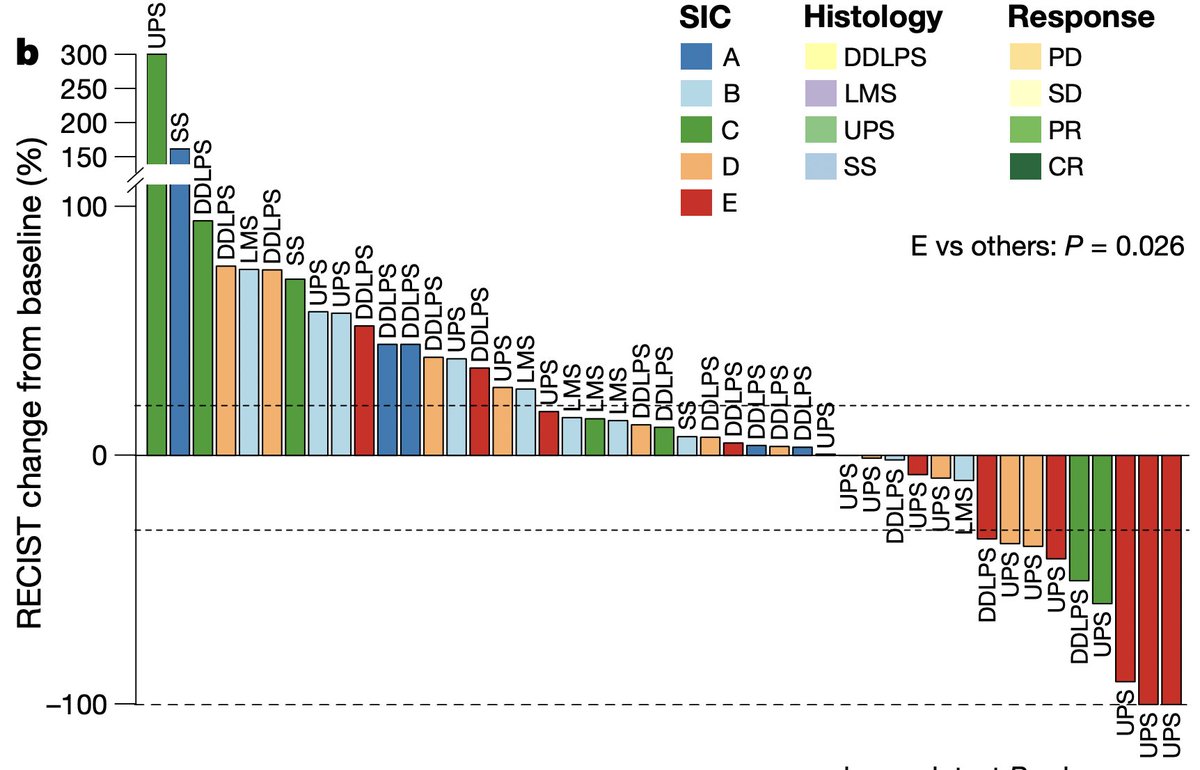
Next, we wanted to see how transplant and primary tumors compared to human sarcomas. @Barzin_ compared our mouse models to human sarcomas using the classification system that @petitprez_f et al published in @nature earlier this year. https://www.nature.com/articles/s41586-019-1906-8
Using CIBERSORTx, we found that transplant tumors resembled highly inflamed “Class E” human sarcomas that are likely to respond to PD-1 blockade. In contrast, primary tumors resembled the majority of human sarcomas, which are immune-low and resistant to PD-1 blockade.
We used single-cell analysis to identify differences in the immune microenvironments of primary and transplant tumors, and we discovered that activated CD8+ T cells, which may mediate transplant tumor cure by PD-1 blockade, are enriched in transplant but not primary tumors.
Transplant tumors growing in syngeneic mice are the most commonly used animal model for preclinical immunotherapy studies, and these findings help to explain why so many immunotherapies that show efficacy in transplant tumor models underperform when translated to cancer patients.
The process of injecting tumor cells to initiate transplant tumors creates an artificial immune response to the injected tumor cells, which primes the immune system to respond to immunotherapy and radiation therapy.
Our study suggests that patients with sarcomas resembling transplant tumors may benefit most from RT and immunotherapy. We hope this work informs design of preclinical experiments to increase successful translation of immunotherapies from preclinical studies to patients.
@DKirschMDPhD's mentorship made it all possible, along with contributions from our co-authors Jon Himes @DukeMSTP, Collin Kent and David Carpenter @DukeRadOnc, @Barzin_ @max_diehn @AshAlizadeh @StanfordCancer and so many more. Hoping the #Duke Kirsch lab fam can celebrate soon 

For more info, check out our Behind the Paper article here: https://go.nature.com/3lUhSuZ

 Read on Twitter
Read on Twitter