Today the @FDA’s vaccine advisory committee is meeting again:this time to consider @moderna_tx’s #vaccine. By the end of the day, the US could be on their way to authorizing a 2nd vaccine. It can’t come soon enough. #Covid19 kills more than 2 ppl, on avg, in the US every minute.
@FDA’s Dr. Doran Fink says they are working with CDC and Alaskan authorities to investigate the cases of 2 adverse reactions to @pfizer's vaccine administered to health care workers. https://www.cnn.com/2020/12/16/health/alaska-allergic-reaction-coronavirus-pfizer-vaccine/index.html
“We don't have enough information to make definitive recommendations one way or another...and will consider whether additional recommendations need to be made.” said @FDA’s Fink. Remember these + the 2 UK reactions are just 4 events out of 10s of thousands of vaccines given.
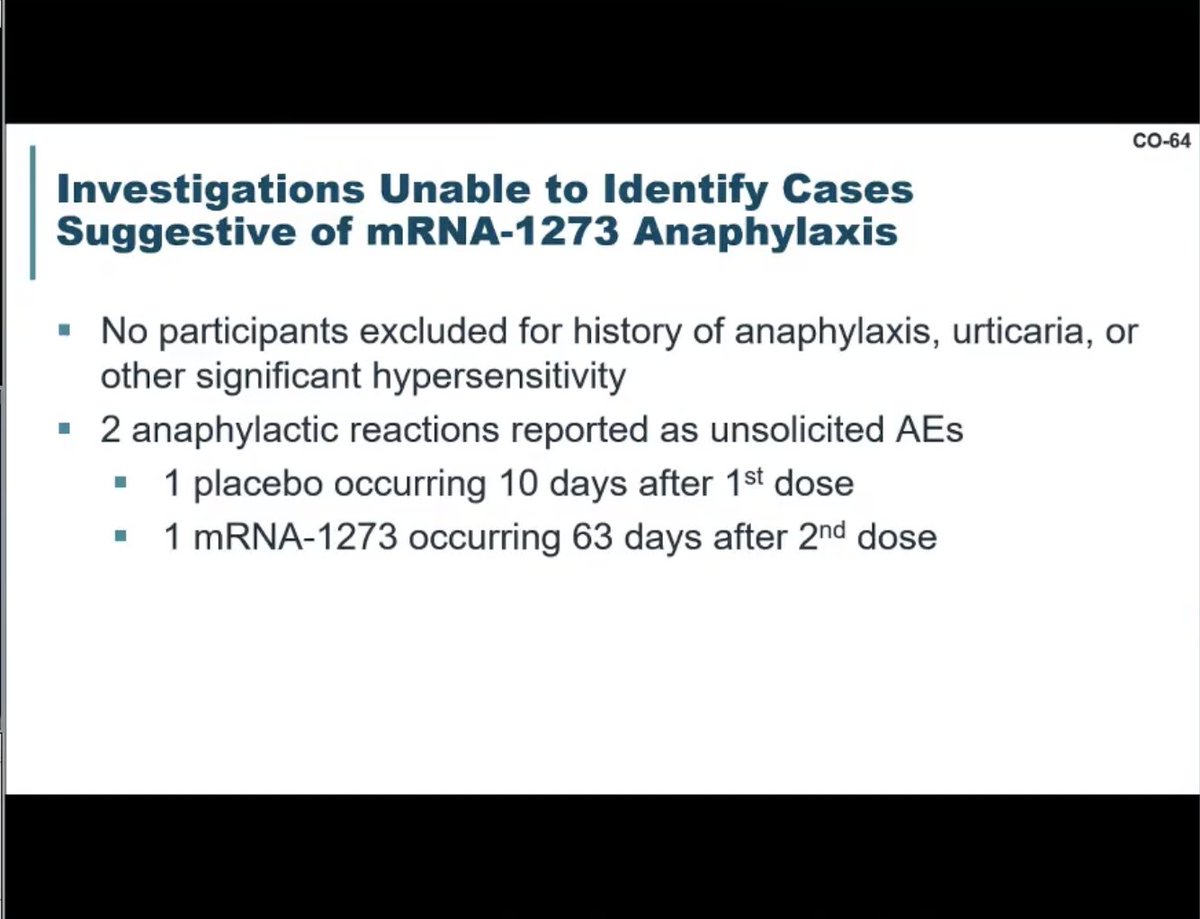
@moderna-tx’s David Martin noted that they looked for any allergic reactions in their trial of about 30,000 people. The only found two cases – neither of them thought to be related to the vaccine.
What does it feel like to take @moderna-tx’s #vaccine? @YasirBatalvi shared with me what it was like to be in the trial. Yes, he was fatigued and had chills – but he says it was well worth it. Vaccines will help, but remember we still have to #WearAMask  https://www.cnn.com/videos/health/2020/12/02/moderna-coronavirus-vaccine-trial-volunteer-side-effects-gupta-pkg-lead-vpx.cnn
https://www.cnn.com/videos/health/2020/12/02/moderna-coronavirus-vaccine-trial-volunteer-side-effects-gupta-pkg-lead-vpx.cnn
 https://www.cnn.com/videos/health/2020/12/02/moderna-coronavirus-vaccine-trial-volunteer-side-effects-gupta-pkg-lead-vpx.cnn
https://www.cnn.com/videos/health/2020/12/02/moderna-coronavirus-vaccine-trial-volunteer-side-effects-gupta-pkg-lead-vpx.cnn
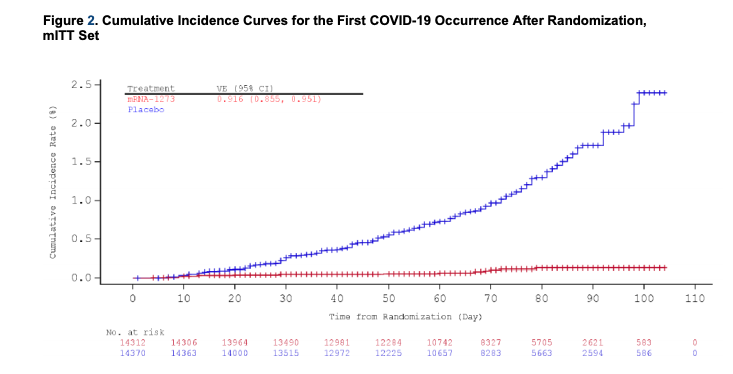
@moderna_tx 's vaccine appears very effective in preventing severe disease. See this graph: the blue line - the rate of cases of #Covid19 in people on placebo, the red line: those on vaccine. Both @FDA and Moderna say 94.5% efficacy is reached at least 14 days after the 2nd dose.
@moderna_tx sent out these letters to trial participants. If the vaccine is authorized, they're offering those on placebo a chance to take vaccine. But that could mean not being able to track long term effects. It’s a point of concern being discussed by the @FDA committee.

 Read on Twitter
Read on Twitter