I am delighted to announce the publication of our latest #teamwild paper, "Mutant huntingtin and neurofilament light have distinct longitudinal dynamics in #Huntingtonsdisease" Rodrigues/Byrne et al 2020 in @ScienceTM:
https://stm.sciencemag.org/content/12/574/eabc2888
#HDResearch
https://stm.sciencemag.org/content/12/574/eabc2888
#HDResearch
This paper signifies the culmination of 5 years of work and collaboration from many people. It highlights the 24-month longitudinal follow-up of the HD-CSF study.
HD-CSF was designed by @profedwild. Blood and cerebrospinal fluid (CSF) collection started in 2016 for baseline and then 2 years later for follow up to test how things change over time in Huntington's disease
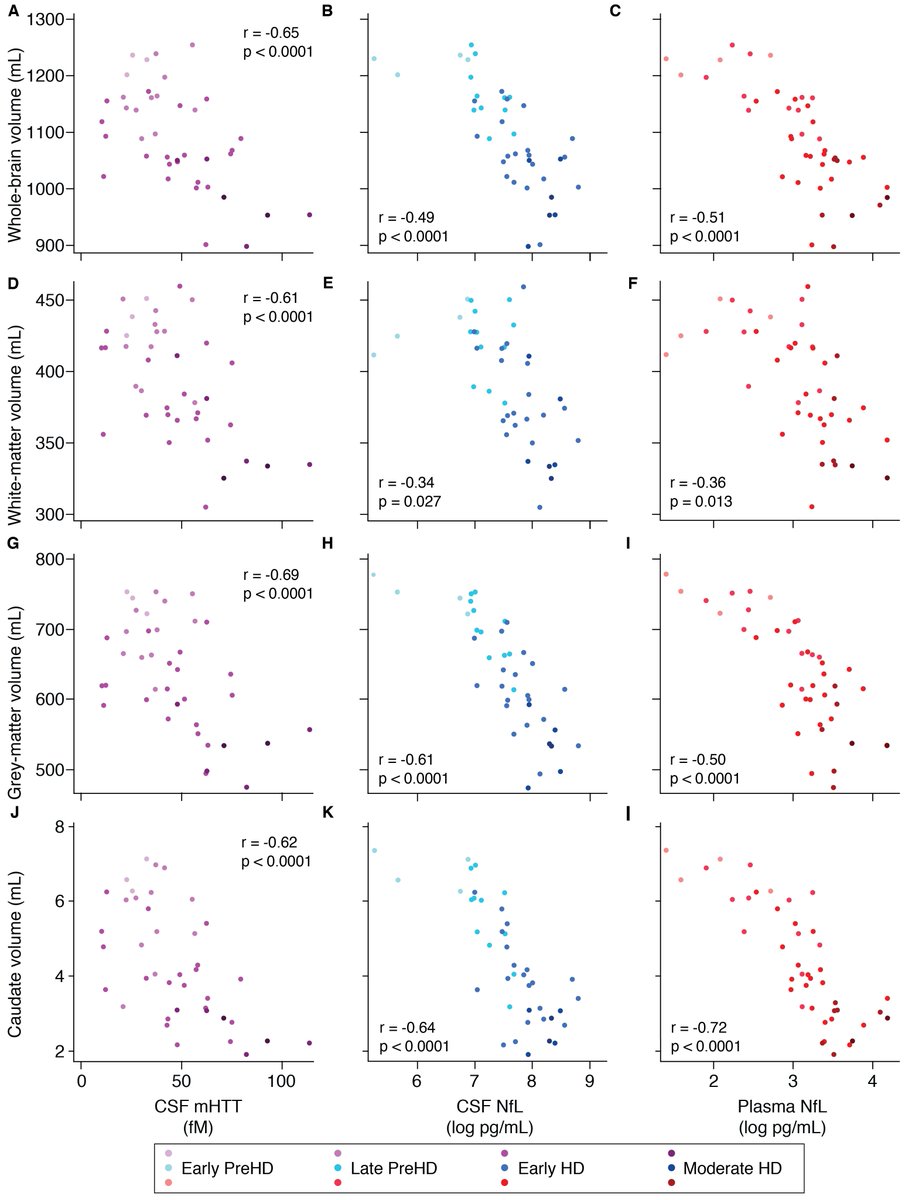
We were particularly interested in mutant huntingtin (mHTT), the harmful protein that damages neurons and causes Huntington's disease; and neurofilament light (NfL), a cytoskeletal protein that's released when neurons are damaged
In the baseline 2018 @ScienceTM paper, we showed that mHTT in CSF and neurofilament in CSF and plasma was increased and correlate with HD measures https://stm.sciencemag.org/content/10/458/eaat7108.short
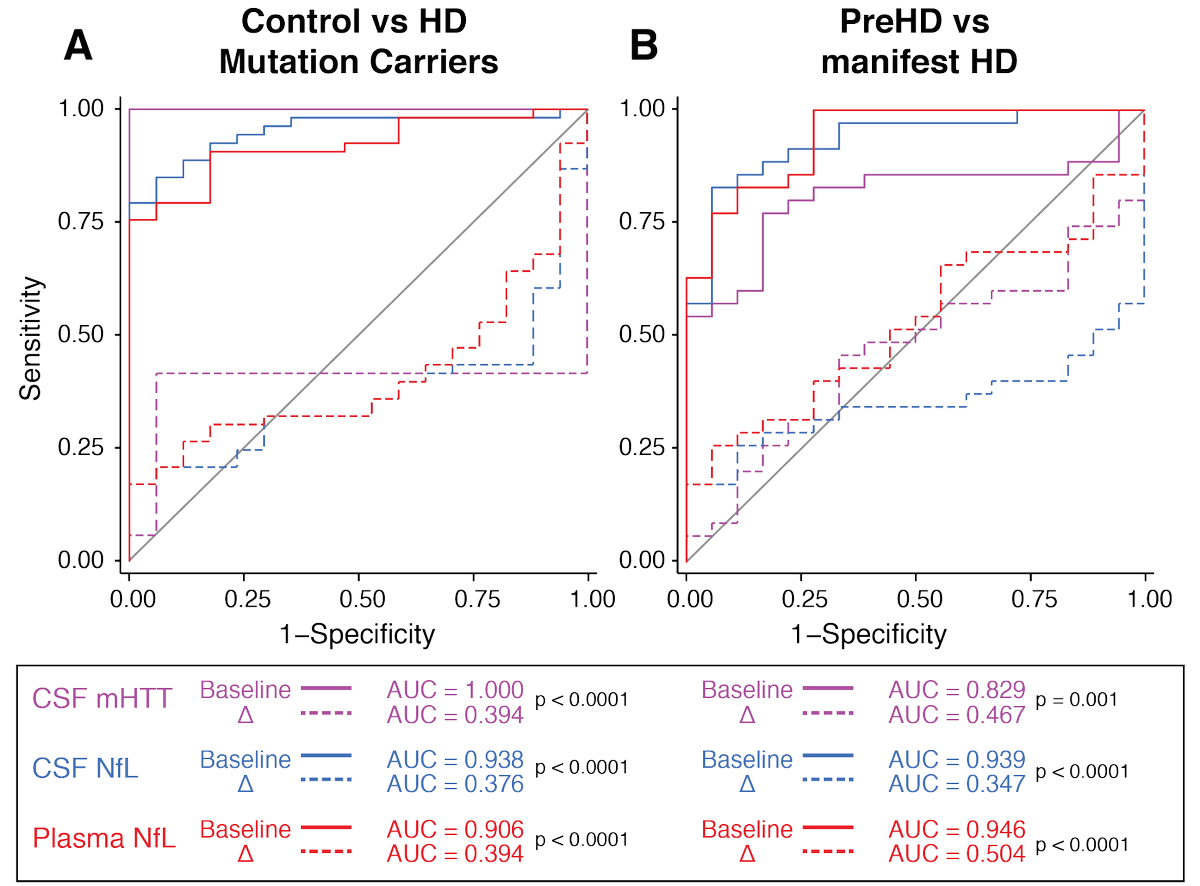
In this new work we can finally examine and compare how each biomarker changed over time and predicts subsequent progression in HD symptoms.
CSF mHTT rises slowly and linearly, while NfL rises in a more sigmoid pattern in HD and is nearly fully divergent from controls for a given age. We modelled each biomarker's relationship with age and CAG repeat count (number of abnormal repeats in the HTT gene)
The two important questions for biomarkers are:
1) What molecule is best for predicting progression?
2) Does change in the molecule over time give us additional information?
1) What molecule is best for predicting progression?
2) Does change in the molecule over time give us additional information?
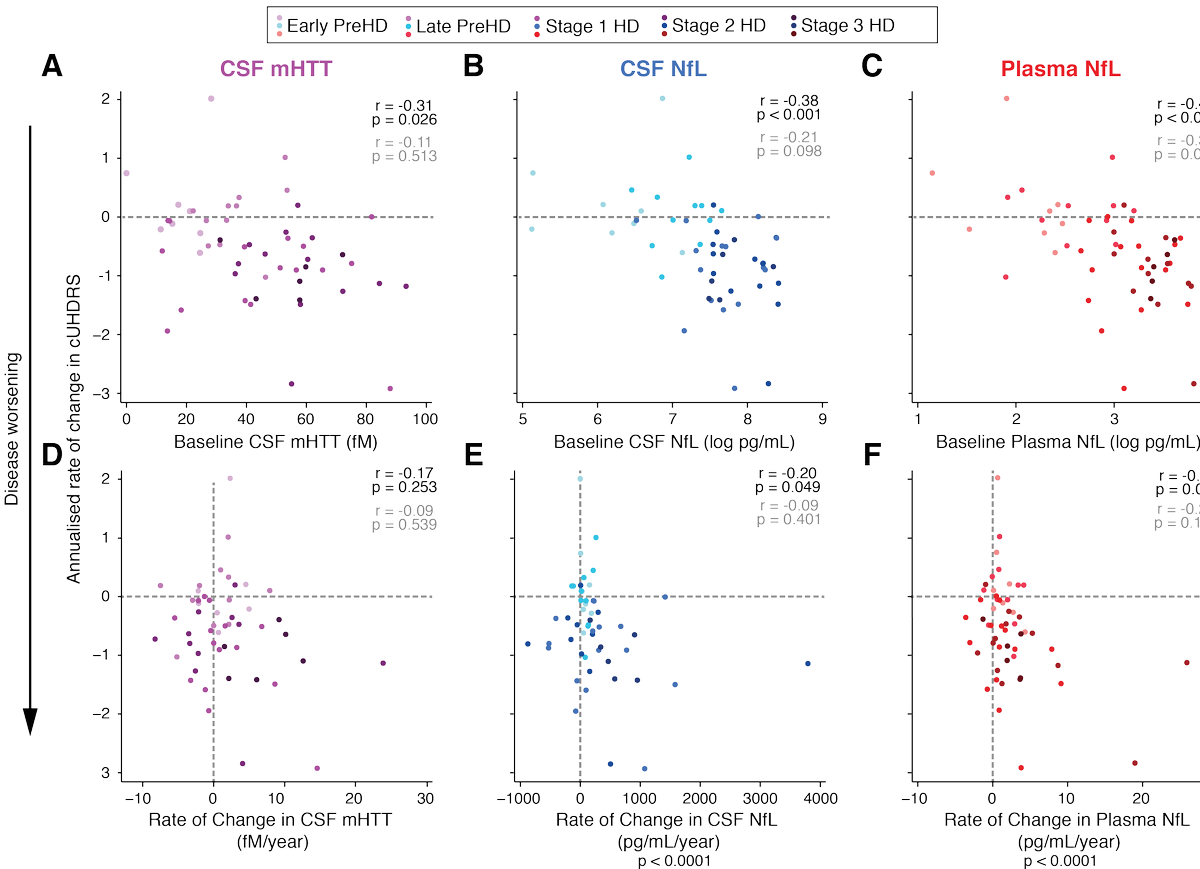
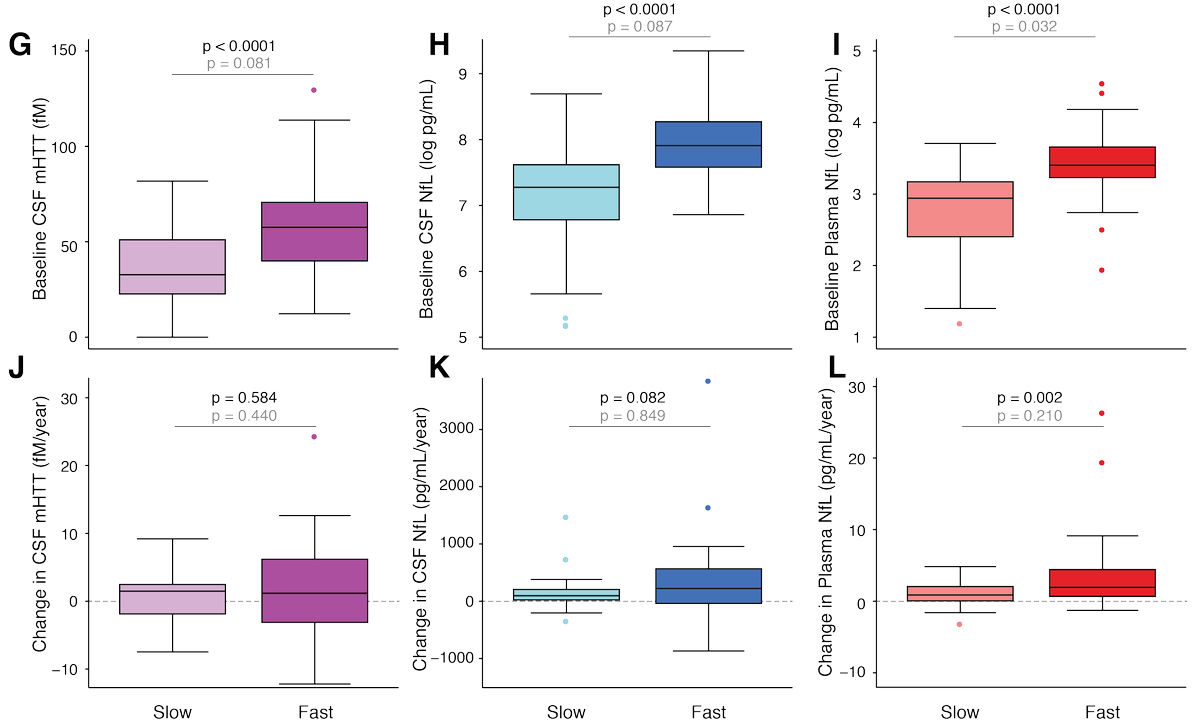
Using the composite Unified Huntington's Disease Rating Scale (cUHDRS) to measure clinical severity, we answer these questions. Top row shows how the baseline values predict subsequent progression; bottom row is how the change values relate to progression over the same period
In brief, the baseline measure is the best predictor of subsequent progression for each biomarker, but the change value probably gives a little extra insight into how a person's brain is coping, especially for NfL.
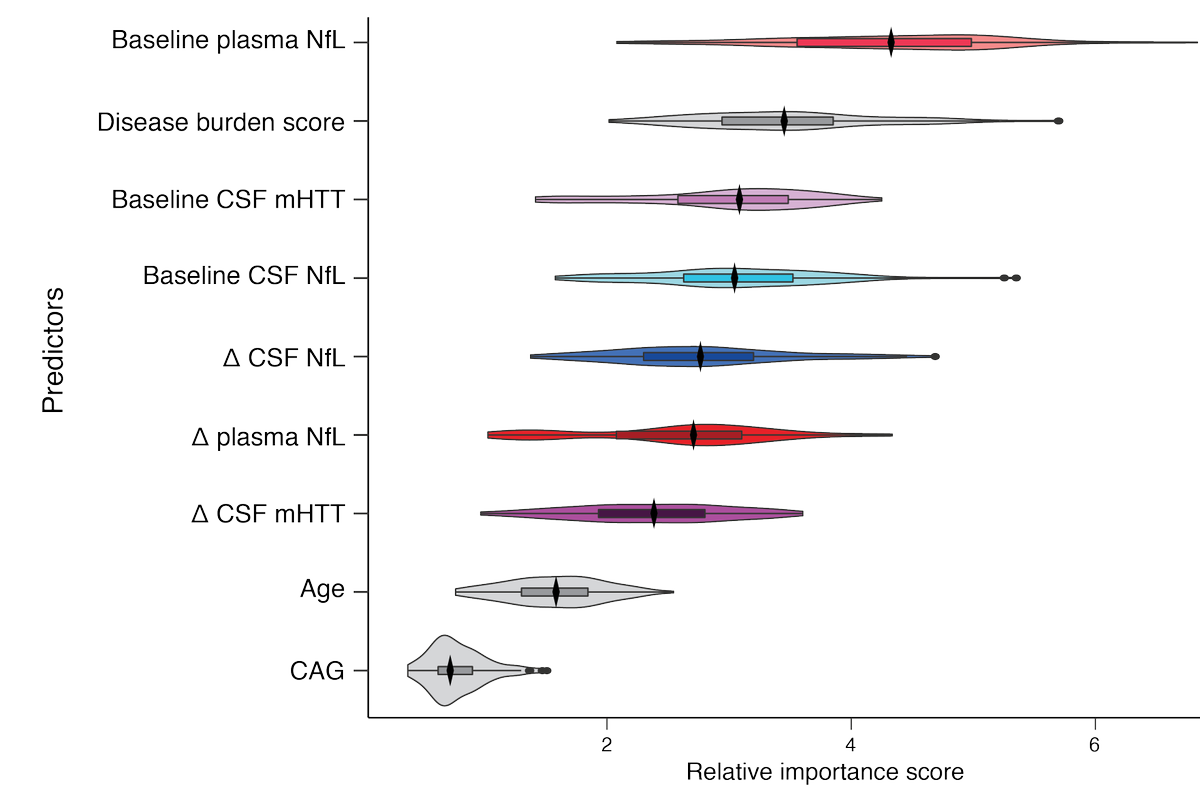
And this is a modelling method called a Random Forest Analysis, which lets us rank the biomarkers by their ability to predict progression. Higher scores = better prediction. Surprisingly, several markers are better predictors than age and CAG count independently.
We ran computer simulations of clinical trials to show how each biomarker would be expected to perform in a trial of a treatment that protects neurons. Each pixel here represents a thousand simulated clinical trials!
We remeasured the biomarkers at baseline and compare batch effects and quantification methods. The NfL re-quantification was highly reliable.
Included in the supplementary: we replicated all the cross-sectional results from our 2018 baseline paper using both the remeasured baseline data and the follow up data. Interestingly, CSF mHTT was now associated with brain volumes.
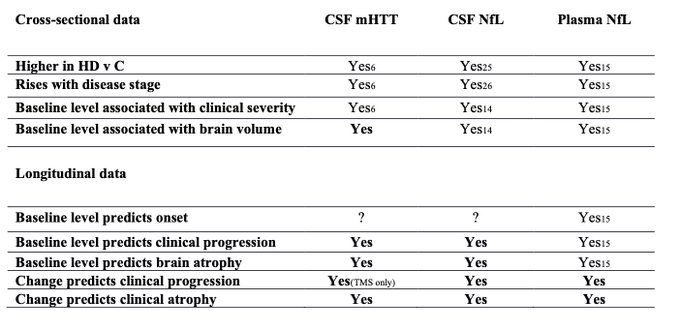
Here we summarise what was already known about mHTT and NfL in HD, and what this longitudinal follow up of the HD-CSF study has added (in bold).
That's the paper in brief - if you have trouble accessing it our preprint is still freely available: https://www.medrxiv.org/content/10.1101/2020.03.31.20045260v1
Thanks to @The_MRC @Roche and CHDI Foundation for funding. And to all co-authors including @filipebrodrigue @LaurenByrne7337 @ProfEdWild @RosannaTortelli @Eileanoir @rachiscahill @WijDr @fishpiechicken @edvJump @Naghmeh7gh @furby10 @ajh1269 @UCLHD
Lastly, but definitely most importantly, we thank all the volunteers who participated, giving much of their time and donating copious bodily fluids. We dedicated this paper to one participant who sadly lost his fight with #Huntingtonsdisease this year #HD #HDResearchheroes

 Read on Twitter
Read on Twitter