I have received my @threadapalooza from @vgr, so let's do this!
A thread about cool materials, and what makes them so. https://twitter.com/vgr/status/1339058822669762560
A thread about cool materials, and what makes them so. https://twitter.com/vgr/status/1339058822669762560
My goal is to hit 100. Although I should acknowledge that last time I only made it to 41.
https://twitter.com/gravity_levity/status/1206018094847680512
Also, I'll post these 5-at-a-time in an attempt to not spam people's timelines too badly
https://twitter.com/gravity_levity/status/1206018094847680512
Also, I'll post these 5-at-a-time in an attempt to not spam people's timelines too badly
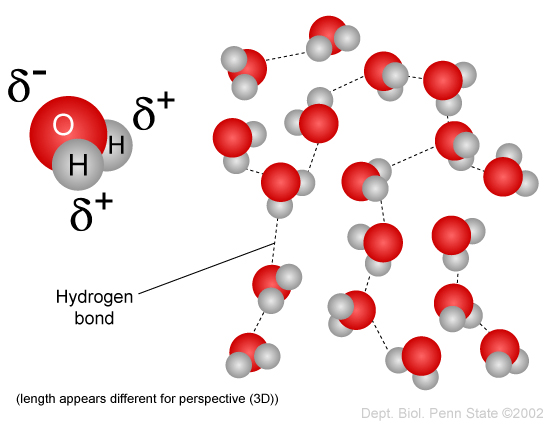
1. We might as well start with WATER.
Water has a million crazy and cool properties, most of which arise from its polar interactions. Water has a huge heat capacity, huge dielectric constant, and dissolves nearly everything.
Water has a million crazy and cool properties, most of which arise from its polar interactions. Water has a huge heat capacity, huge dielectric constant, and dissolves nearly everything.
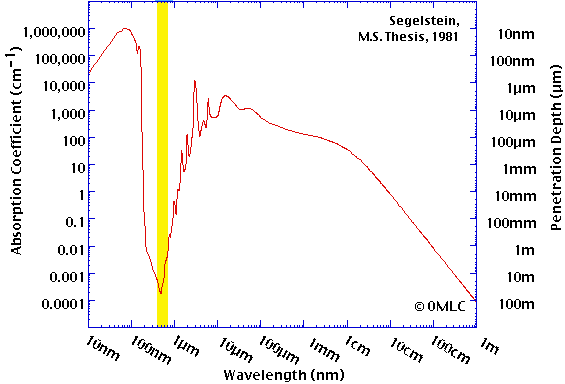
Also, water is essentially opaque to all light except a narrow band of wavelengths that happens to correspond to the ones used by our eyes (shown in yellow)
2. ICE is one of the very rare materials that is less dense than its liquid form. Lucky for us, or else water that freezes in a lake would sink to the bottom and never thaw.
Also, it has 18 (!) distinct solid phases, as well as an "amorphous solid" phase.
Also, it has 18 (!) distinct solid phases, as well as an "amorphous solid" phase.
You know the Vonnegut novel "Cat's Cradle"? It's about a disovered form of ice that's solid at room temperature, and therefore can act as a seed that freezes all water and destroys the world. The Nobel-winning physical chemist Irving Langmuir came up with that premise.
Side note: Ice doesn't heat well in a microwave, because microwaves target an electromagnetic resonance that appears in liquid but not solid water molecules.
So if you microwave something half-frozen, you may find that one side gets boiling hot while the other is still frozen.
So if you microwave something half-frozen, you may find that one side gets boiling hot while the other is still frozen.
3. RUBBER is made of long polymers (molecular chains) that are all tangled together -- it's their knottedness that makes the material solid-seeming yet stretchy.
Rubber heats/cools as you stretch/relax it, because the polymers are changing their (configurational) entropy.
Rubber heats/cools as you stretch/relax it, because the polymers are changing their (configurational) entropy.
Google "rubber band heat engine" to get some cool demos of this effect and its consequences. The physics goes surprisingly deep! I once heard a talk about rubber bands from Chris Jarzynski, one of our greatest modern theorists of thermodynamics.
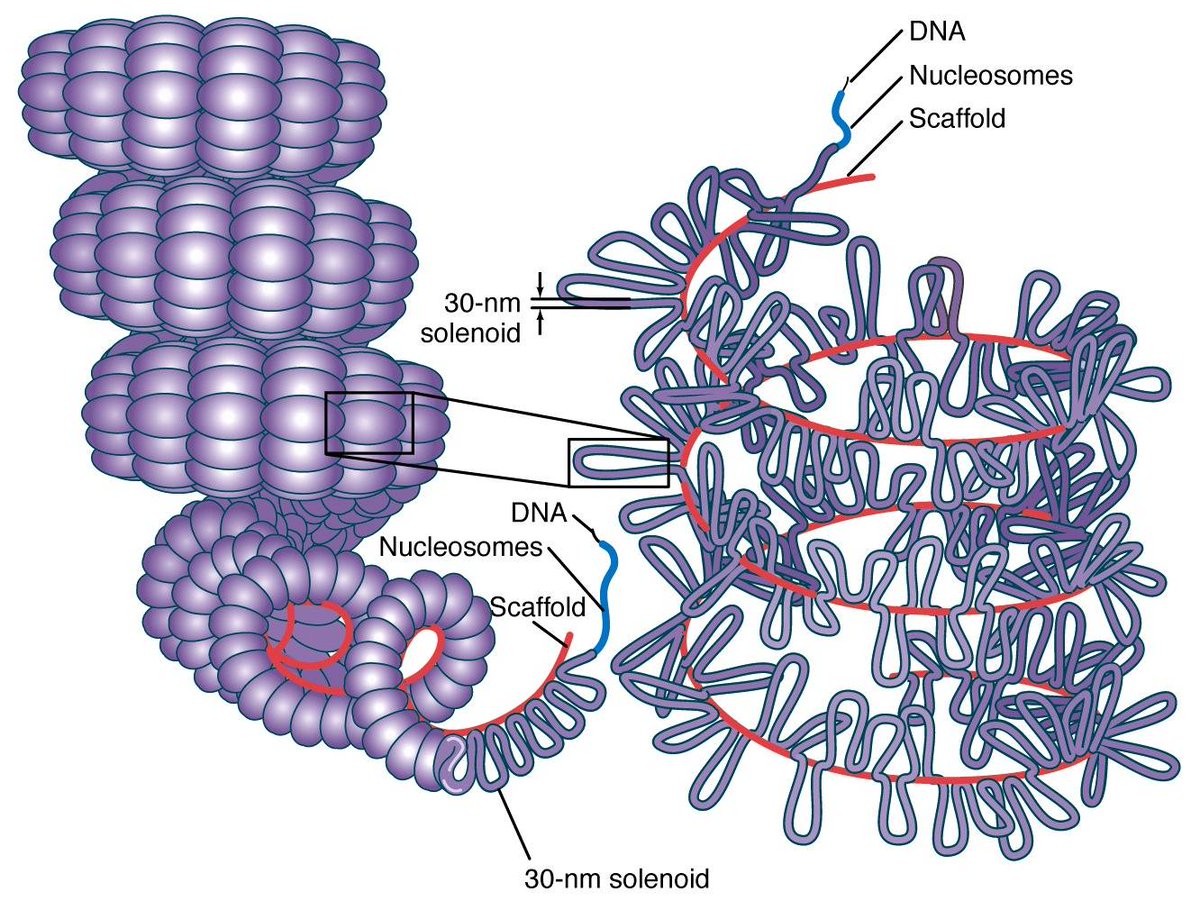
4. DNA (as a material, rather than as information storage) is crazy. You have a 3-meter-long strand packed inside the 0.01 mm - sized nuceleolus of each cell. Somehow it all has to get accessed, unzipped, transcribed, and repackaged all the time without ever getting tangled.
Think about how easy it is to get even your headphone cables tangled up, and you'll see how implausible this seems. But it works somehow; nature is just much cleverer than we are about its packaging.
Also, DNA as a polymer is actually quite rigid due to its double-helix structure. But it also carries an enormous electric charge, and so it can be bent into place by very strong Coulomb forces.
5. MERCURY
There's something about liquid metals that seems implausible. Our understanding of electrical conduction is generally based on the idea of electrons moving through an orderly environment. So to have a random/liquid environment with metallicity feels supernatural.
There's something about liquid metals that seems implausible. Our understanding of electrical conduction is generally based on the idea of electrons moving through an orderly environment. So to have a random/liquid environment with metallicity feels supernatural.
Mercury was also the first superconductor! Seeing the electrical resistance of an object drop literally to zero below a certain temperature was such a shock that we in physics are still obsessed with it > 100 years later.
6. LIQUID HELIUM(-4)
What happens when you have a material made of bosonic atoms that remains liquid all the way down to zero temperature?
It becomes a "superfluid" and does crazy things like:
- climb the walls of its container and empty itself onto the floor ("fountain effect")
What happens when you have a material made of bosonic atoms that remains liquid all the way down to zero temperature?
It becomes a "superfluid" and does crazy things like:
- climb the walls of its container and empty itself onto the floor ("fountain effect")
- If you put it in a bucket, and then start rotating the bucket, the superfluid will remain completely still. Then, at a critical rotation frequency, the fluid will start to develop quantized vortices.
7. LIQUID HELIUM-3
This is a distinct form of helium, purified so that it only has fermionic atoms (1 neutron per atom instead of 2). It isn't supposed to become a superfluid because it is made of fermions, which cannot undergo Bose condensation. But He-3 goes superfluid anyway.
This is a distinct form of helium, purified so that it only has fermionic atoms (1 neutron per atom instead of 2). It isn't supposed to become a superfluid because it is made of fermions, which cannot undergo Bose condensation. But He-3 goes superfluid anyway.
Somehow, two fermionic atoms bind to each other (in a process analogous to superconductivity, where two electrons bind together), to make "composite bosons". These composite bosons form a superfluid.
Another crazy thing about He-3: at low-T its solid phase has more entropy than its liquid phase. (I know, this violates everything your high school chemistry teacher taught you about what entropy is)
This means that, under the right conditions, He-3 _freezes_ when you heat it up
This means that, under the right conditions, He-3 _freezes_ when you heat it up
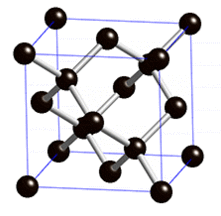
8. IRON
Magnets are crazy in general (as noted by a certain clown posse). There is actually a very general proof that classical physics does not allow magnetism to exist: https://thiscondensedlife.wordpress.com/2016/10/29/bohr-van-leeuwen-theorem-and-micromacro-disconnect/
So magnets are macroscopic manifestations of quantum mechanics
Magnets are crazy in general (as noted by a certain clown posse). There is actually a very general proof that classical physics does not allow magnetism to exist: https://thiscondensedlife.wordpress.com/2016/10/29/bohr-van-leeuwen-theorem-and-micromacro-disconnect/
So magnets are macroscopic manifestations of quantum mechanics
Iron is especially difficult because it contains both magnetism and freely-conducting electrons. This is something we still don't have a good theory of. So far our theories of magnetism are mostly built to describe little bar magnets (the electrons themselves) frozen in place.
(You can read my intro explainer to where magnets come from here: https://gravityandlevity.wordpress.com/2015/04/19/where-does-magnetism-come-from/
Anyone who claims to explain magnetism without talking about the Pauli exclusion principle [and the "exchange interaction"] is ripping you off.)
Anyone who claims to explain magnetism without talking about the Pauli exclusion principle [and the "exchange interaction"] is ripping you off.)
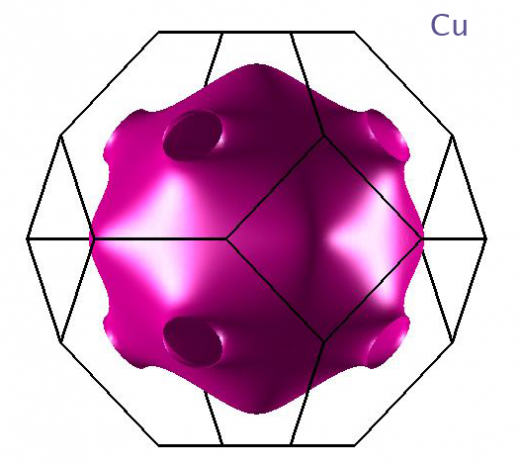
9. COPPER
To me, the funniest thing about copper is always that it turns green when it rusts. So our Statue of Libery started out looking like a flaming beacon of hope and quickly turned into Kermit the frog.
To me, the funniest thing about copper is always that it turns green when it rusts. So our Statue of Libery started out looking like a flaming beacon of hope and quickly turned into Kermit the frog.
Here's something else weird about copper. Electrons in its interior are flying around at (literally) a million miles per hour. If you take a census of their momentum, you'll find that all the values sit neatly inside a geometric surface ("Fermi surface") that looks like this:
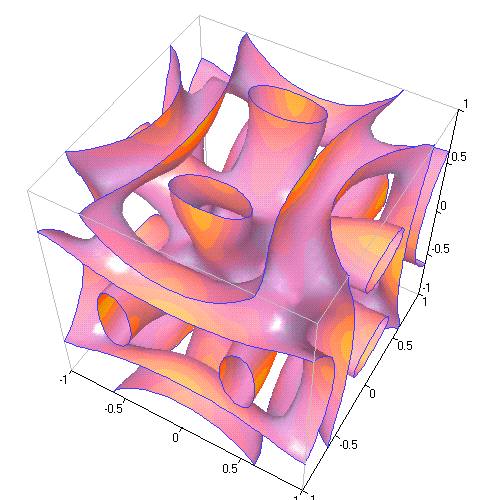
10. BUCKMINSTERFULLERENE
"Buckyballs" are those little soccer ball shapes made of 60 carbon atoms, which you've likely heard of. But I bet you didn't know that, if you make a 3D crystal of buckyballs the Fermi surface looks like this:
"Buckyballs" are those little soccer ball shapes made of 60 carbon atoms, which you've likely heard of. But I bet you didn't know that, if you make a 3D crystal of buckyballs the Fermi surface looks like this:
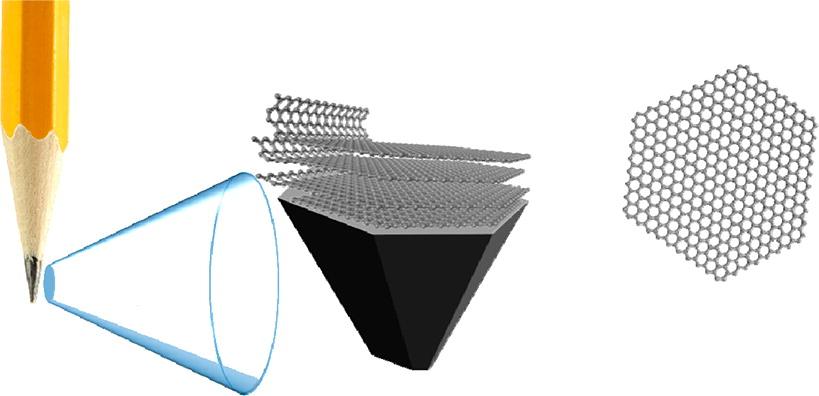
11. GRAPHITE
Graphite is so good for making pencils because it is made from flaky layers, that are individually strong but only weakly bound to each other. When you scrape the pencil over the paper those layers flake off onto the paper, leaving a dark trail.
Graphite is so good for making pencils because it is made from flaky layers, that are individually strong but only weakly bound to each other. When you scrape the pencil over the paper those layers flake off onto the paper, leaving a dark trail.
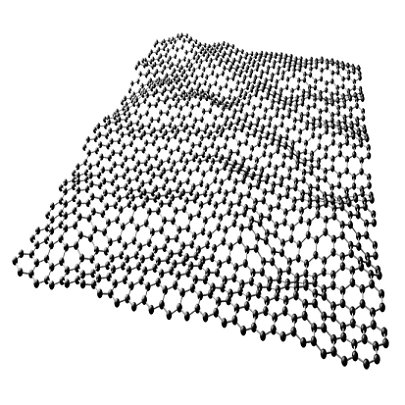
12. GRAPHENE
It's hard to know what to say about graphene, the 2D wonder material made of a single-atom-thick net of carbon atoms. At this point we've been promised everything from touch screens to bulletproof vests to solar panels to condoms.
https://en.wikipedia.org/wiki/Potential_applications_of_graphene
It's hard to know what to say about graphene, the 2D wonder material made of a single-atom-thick net of carbon atoms. At this point we've been promised everything from touch screens to bulletproof vests to solar panels to condoms.
https://en.wikipedia.org/wiki/Potential_applications_of_graphene
But here's something you probably didn't know about graphene: It absorbs exactly πα ≈ 2.3% of the light that hits it, where α ≈ 1/137 is the fine structure constant.
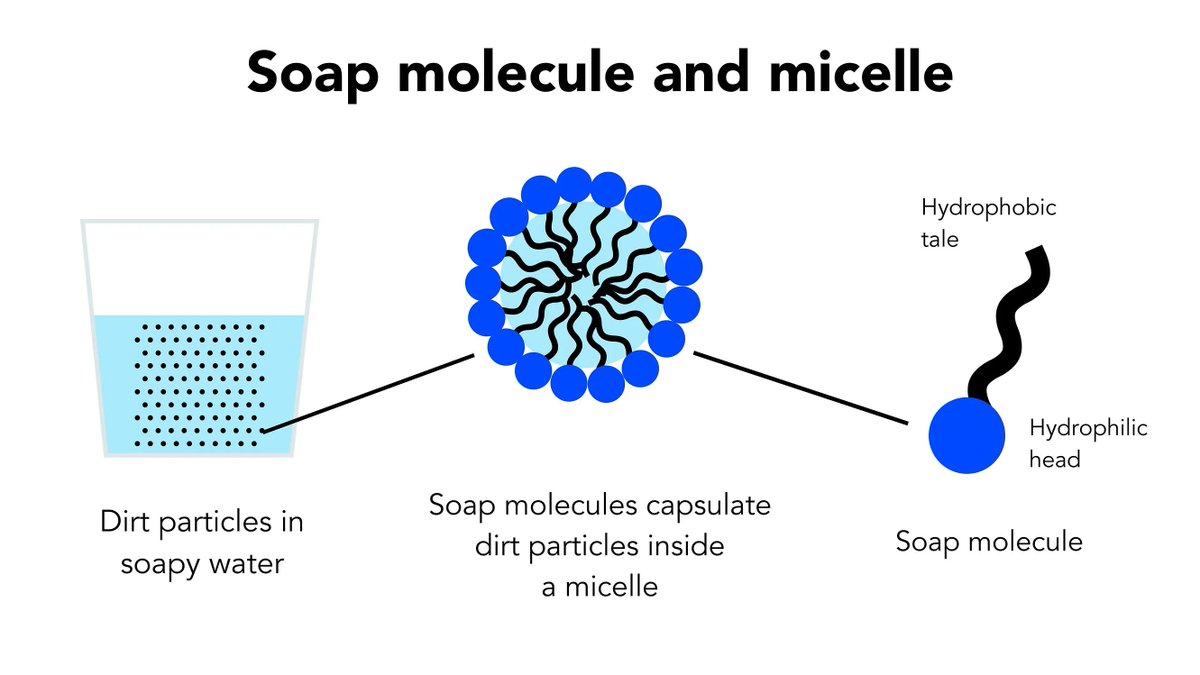
13. SOAP
What a very clever idea soap is. You make a long molecule with a hydrophobic end (sticks to stuff on your hands) and a hydrophillic end (sticks to water). Then running water pulls things off your hands.
What a very clever idea soap is. You make a long molecule with a hydrophobic end (sticks to stuff on your hands) and a hydrophillic end (sticks to water). Then running water pulls things off your hands.
14. PLASTIC
Plastic might be the most useful and pervasive material in our lives. But what a mess it is to understand at the micro-level: some tangled matt of polymers, with no easily-understandable structure or emergent properties.
Plastic might be the most useful and pervasive material in our lives. But what a mess it is to understand at the micro-level: some tangled matt of polymers, with no easily-understandable structure or emergent properties.
I think of plastic as an absolute (and rare) triumph of embracing non-legibility in science.
( https://www.ribbonfarm.com/2010/07/26/a-big-little-idea-called-legibility/)
( https://www.ribbonfarm.com/2010/07/26/a-big-little-idea-called-legibility/)
15. VELCRO
If you've never seen velcro under a microscope, you should. It's literally just tiny hooks grabbing tiny loops. Who had the audacity to invent that?
If you've never seen velcro under a microscope, you should. It's literally just tiny hooks grabbing tiny loops. Who had the audacity to invent that?
17. HYDROGEN GAS
Hydrogen was literally the first atom that we understood in the quantum mechanical sense, and in that sense maybe hydrogen gas is the "simplest" material of all.
But what a cool moment it is when you first see the atomic lines of hydrogen gas for yourself.
Hydrogen was literally the first atom that we understood in the quantum mechanical sense, and in that sense maybe hydrogen gas is the "simplest" material of all.
But what a cool moment it is when you first see the atomic lines of hydrogen gas for yourself.
18. METALLIC HYDROGEN
It has been predicted since the 1930s that, at high enough pressure, hydrogen gas could be compressed into a crystalline form. This crystal should be a very high-temperature superconductor, and finding it has been a huge goal for a long time.
It has been predicted since the 1930s that, at high enough pressure, hydrogen gas could be compressed into a crystalline form. This crystal should be a very high-temperature superconductor, and finding it has been a huge goal for a long time.
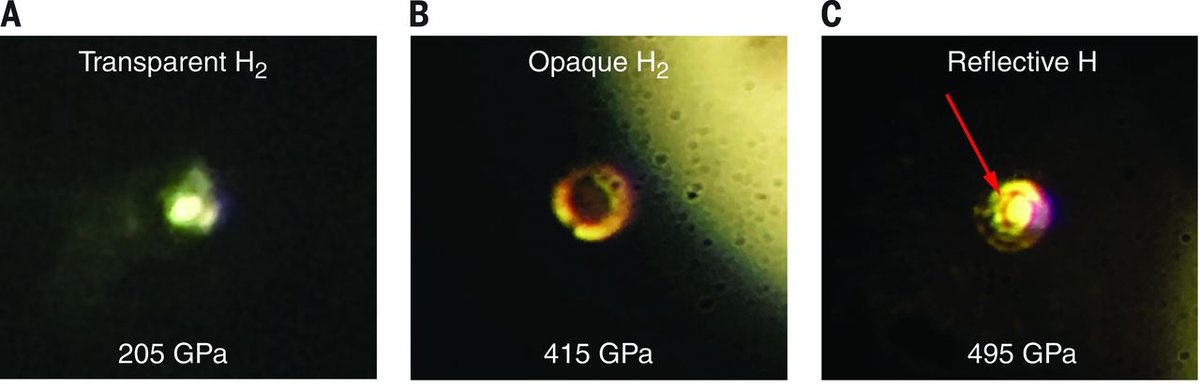
In 2017 a group at Harvard published a Science paper claiming to have created metallic hydrogen by achieving a pressure of ~500 GPa (5000x the pressure at the deepest point of the ocean).
One of their key pieces of evidence was an iPhone photo of something becoming shiny:
One of their key pieces of evidence was an iPhone photo of something becoming shiny:
(As far as I can tell, most people don't believe this claim.)
19. GOLD is so valuable largely because it is so chemically inert. This also makes it widely used in experiments when you want something that will just act as a metal without doing anything annoying
But apparently gold also has topological surface states! https://www.nature.com/articles/ncomms10167
But apparently gold also has topological surface states! https://www.nature.com/articles/ncomms10167
20. SILICON is literally just sand, and people who studied silicon used to get made fun of by other physicsts for "studying dirt". But now modern life revolves around semiconductors.
The big deal about silicon (and other semiconductors) is that they have allowed and disallowed bands of energy that can carry current. By controllably injecting electrons into those bands, you can control whether something acts like a metal or insulator, and hence digital logic.
21. SAND PILES
Here's something interesting about a pile of sand:
Every material has its own characteristic "angle of respose", which is the angle it makes with the ground when you pile it up.
https://en.wikipedia.org/wiki/Angle_of_repose
Here's something interesting about a pile of sand:
Every material has its own characteristic "angle of respose", which is the angle it makes with the ground when you pile it up.
https://en.wikipedia.org/wiki/Angle_of_repose
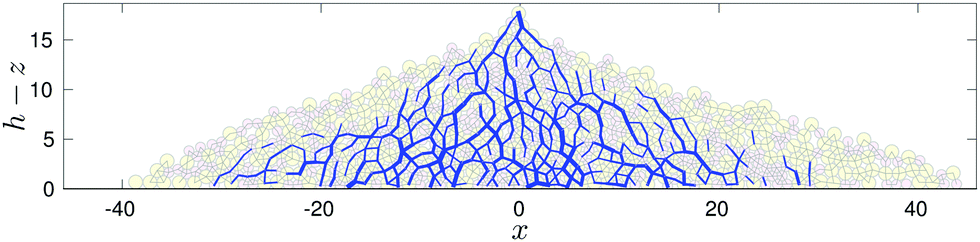
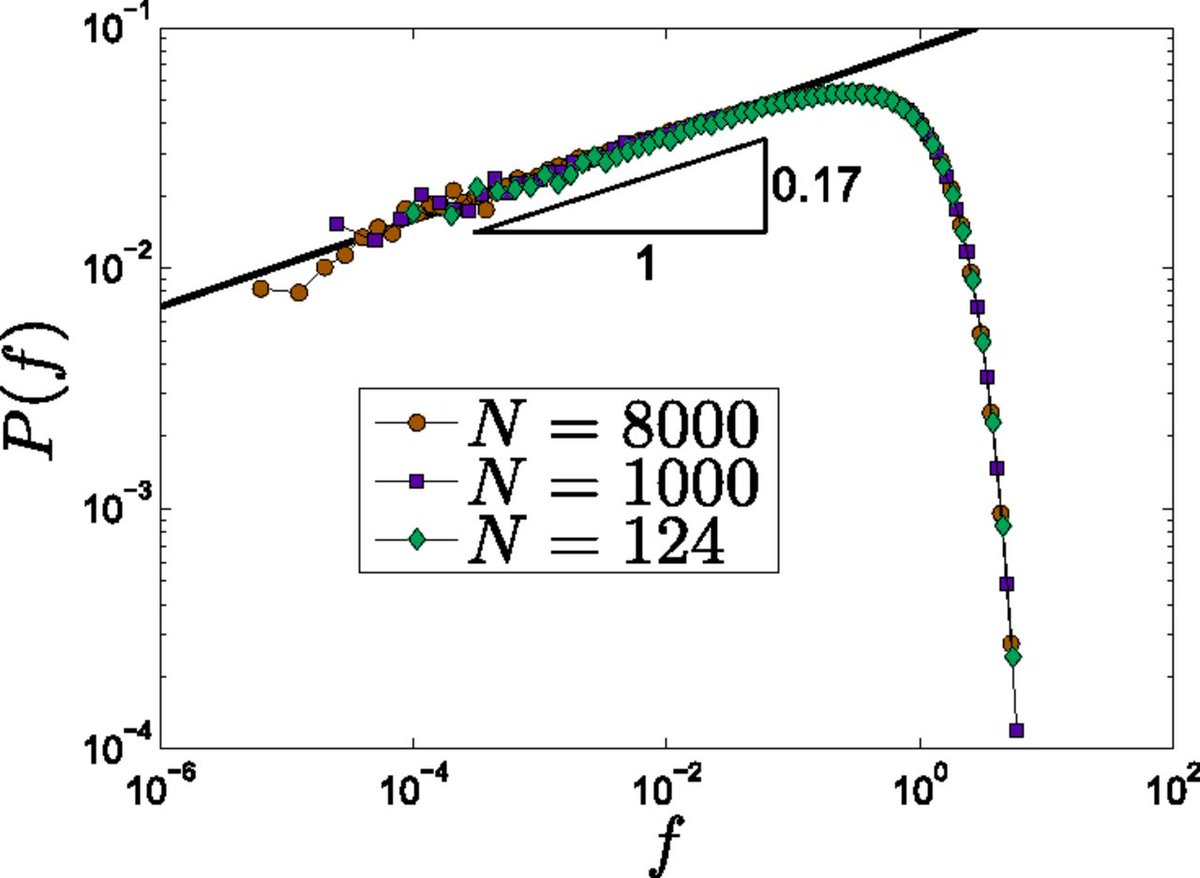
The mechanical properties of sand piles are generally mysterious. The pile is supported by long "force chains" with fractal structure. And if you make a histogram of the force F on each sand grain, there is a mysterious power-law vanishing of the probability when F gets small.
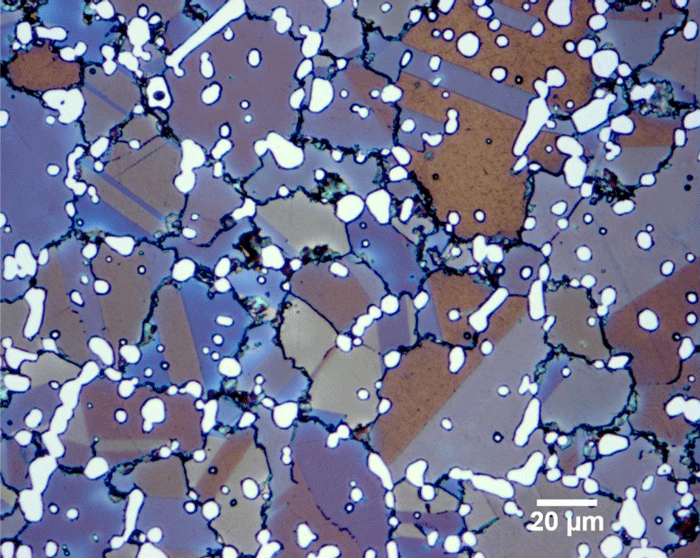
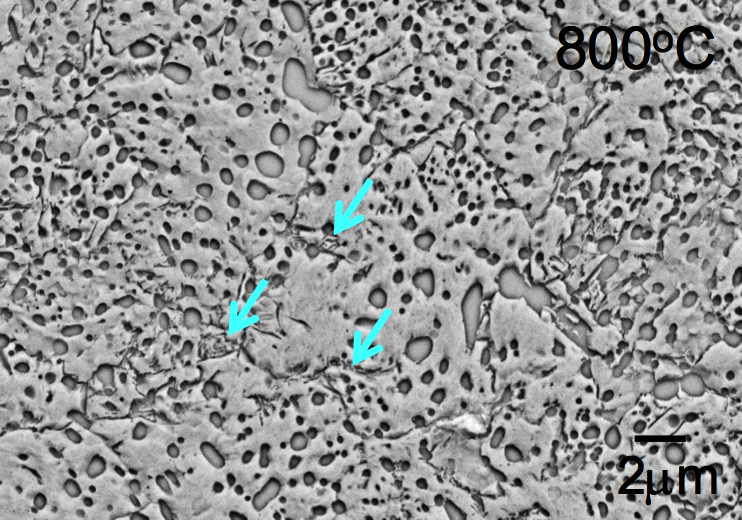
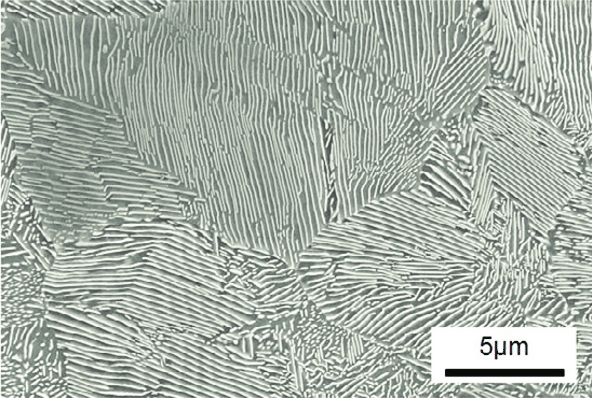
22. STEEL is just iron + carbon melted together, but it turns out that there is a whole zoology of very different things you can get from this process.
23. As a digression, let me go on a rant about how much I dislike the high school factoid "actually, there are four forms of matter: solid, liquid, gas, and PLASMA".
For one thing: if you're going to dip into esoterica, there are many more than four forms of matter.
For one thing: if you're going to dip into esoterica, there are many more than four forms of matter.
But also, plasma is just gas for which the electrons have become dissociated from their nuclei. How is that a "different form of matter"?
It's an empty factoid. Don't give in to the lobbying of big plasma.
/rant
It's an empty factoid. Don't give in to the lobbying of big plasma.
/rant
24. DIAMOND as a form of carbon is technically unstable, in the sense that it has a higher energy than graphite. But in practice it is extremely stable, rigid, and inert both electrically and optically. (Its electron states are very tightly bound in place.)
A funny thing about diamond: it is a very good heat conductor (at room temperature). Most good heat conductors are metals, because the heat is carried by free-flowing electrons. But in diamond there are no such free electrons.
Instead, the heat in diamond is carried by sound waves. The speed of sound in diamond is unusually fast because of the light mass of the carbon atoms and their very strong interactions with each other.
(A mattress salesman once tried to sell me a mattress that had crushed up diamonds in it, because he said that "diamonds are the coldest substance known to man".
I guess you know what they say about just a little bit of knowledge )
)
I guess you know what they say about just a little bit of knowledge
 )
)
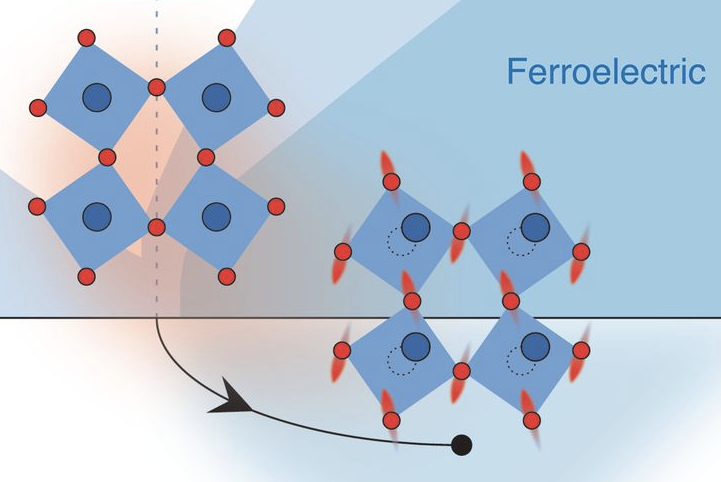
25. STRONTIUM TITANATE is a synthetic diamond (more sparkly, less hard, much cheaper). Its dielectric constant is enormous: ~20,000. This means that an electric charge Q inside it only produces the force of a charge Q/20,000. Nearly all its charge is "cancelled".
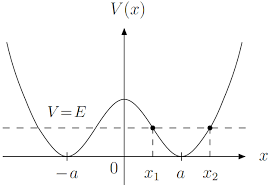
This happens for a kind of cool reason. The lattice of strontium atoms is actually unstable in a classical sense. The energy of the system is lowered if the strontium atom falls into one of several equivalent, off-center potential wells (a "ferroelectric transition").
But this instability doesn't actually happen, because the strontium atom is able to _quantum tunnnel_ between different potential wells. It therefore sits in all wells simultaneously.
A very small electric field is able to push a strontium atom into just one well or the other (since that's what it kind of wanted to do anyway). The lattice is therefore very easily polarizable, and this allows it to "cancel" an external charge, i.e. a huge dielectric constant
26. Let's have a little more fun with this thread.
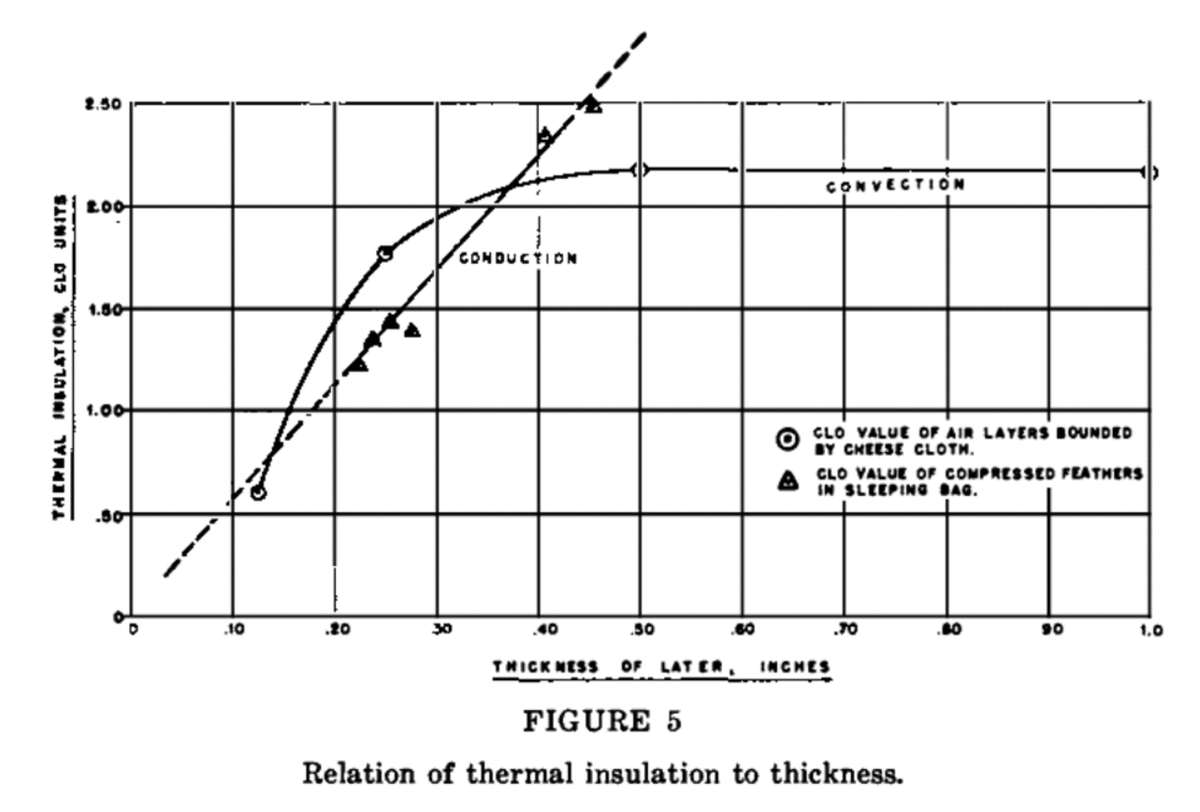
It's hard to think of a more amazing material than FEATHERS. Extremely light, yet still strong enough to withstand huge stresses and sufficiently impermeable to air that they can serve as excellent thermal insulation.
It's hard to think of a more amazing material than FEATHERS. Extremely light, yet still strong enough to withstand huge stresses and sufficiently impermeable to air that they can serve as excellent thermal insulation.
The US Army commissioned a study of the mechanical properties of feathers during WWII, and concluded that they were the only material that had a sufficiently low thermal conductivity, low weight, and high ratio (uncompressed volume)/(compressed volume) https://www.nap.edu/read/18880/chapter/9
The iridescent color you sometimes see in feathers results from "structural color": a micro-layering of different dielectric layers at the scale of the wavelength of light. This leads to certain wavelengths being selectively reflected.
https://academy.allaboutbirds.org/how-birds-make-colorful-feathers/
https://academy.allaboutbirds.org/how-birds-make-colorful-feathers/
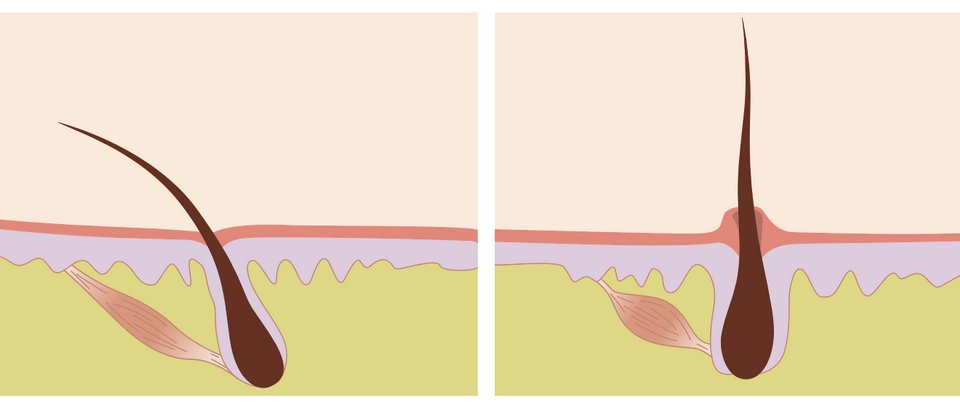
27. FUR or hair is similarly amazing, with similar properties (this photo is from a honeybee).
Fur is less structural and has a more dynamic relationship with thermal regulation. For example, one of the reasons it stands on end is to increase the rate of evaporation.
Fur is less structural and has a more dynamic relationship with thermal regulation. For example, one of the reasons it stands on end is to increase the rate of evaporation.
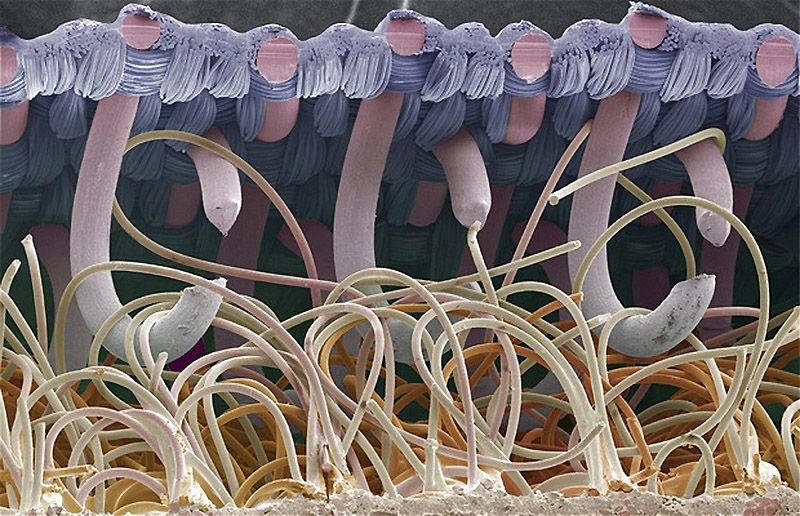
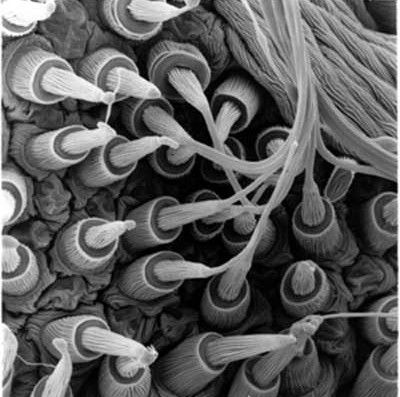
28. SPIDER SILK is a wonder material. It is extremely light: a strand long enough to circle the earth would weight less than half a kg. Its tensile strength per unit weight is about five times larger than steel.
(photo of spider silk glands)
(photo of spider silk glands)
Where spider silk really excels, though, is in its "toughness": by stretching significantly in response to an applied force, it can absorb a huge amount of energy without breaking.
29. Of course, all three of the last entries are really examples of the wonders of KERATIN. Feathers, fur, silk, fingernails, scales, horns, and hooves are all made from long strands of the keratin polymer bundled up into fibers or sheets.
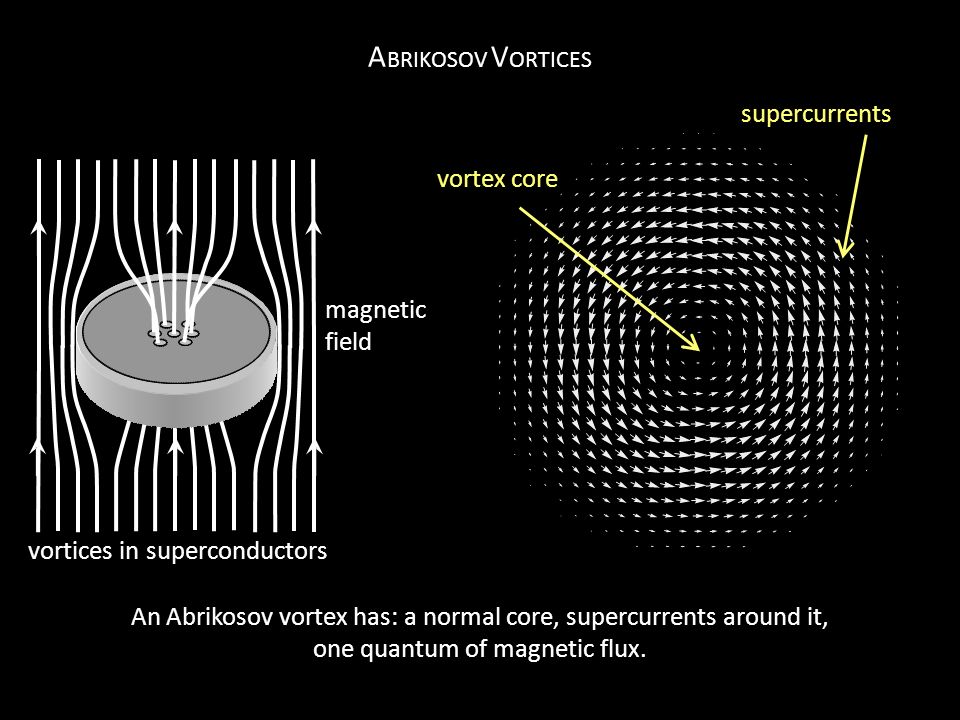
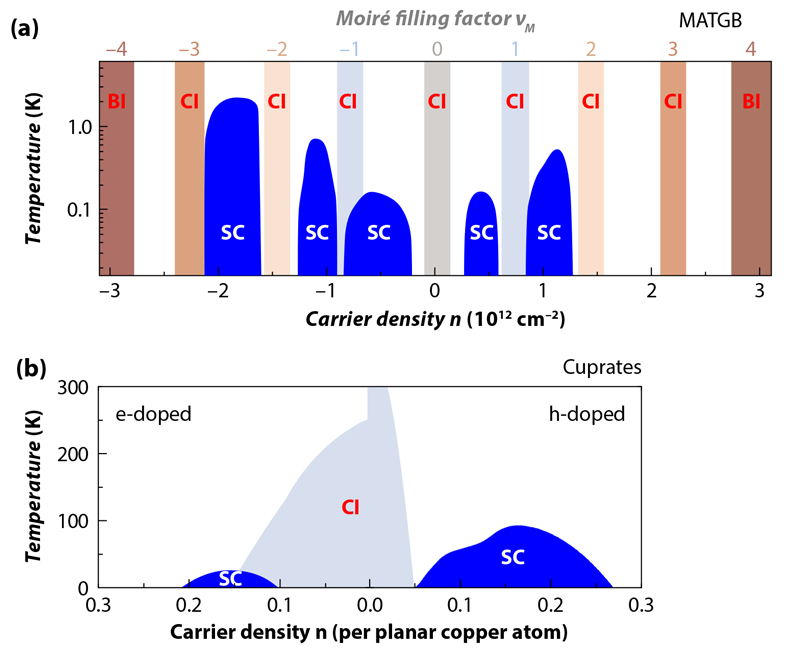
30. The CUPRATE SUPERCONDUCTORS have been an obsession in physics + materials science for 34 years. To see something go from moderately-large resistance to literally zero resistance just by pouring liquid nitrogen on it always feels supernatural.
Dramatic public demos of superconductivity always focus on magnetic levitation. But people rarely explain how levitation works.
The key idea is that superconductors repel magnetic field (they produce an equal + opposite internal field to cancel it).
...
The key idea is that superconductors repel magnetic field (they produce an equal + opposite internal field to cancel it).
...
But if the applied magnetic field is strong, this process is very energetically expensive. So in certain superconductors, the "superelectrons" part like the Red Sea in order to allow magnetic field to pass through the material in distinct "flux tubes".
When you see levitation, it's because these flux tubes are getting "pinned" to some defect in the material, which superelectrons were trying to avoid anyway. There is thus a force that resists sliding the flux tube to the side, and the material itself gets pinned in space.
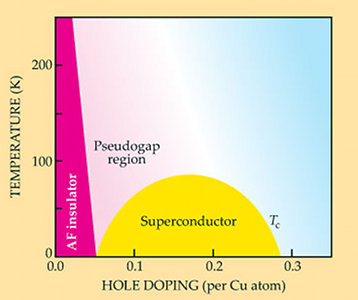
One of the most mysterious things about the high-temperature superconductors is that they arise out of a state that isn't even a good conductor. In fact, you make a cuprate superconductor by starting with an insulator and injecting just ~0.1 additional electrons per Cu atom.
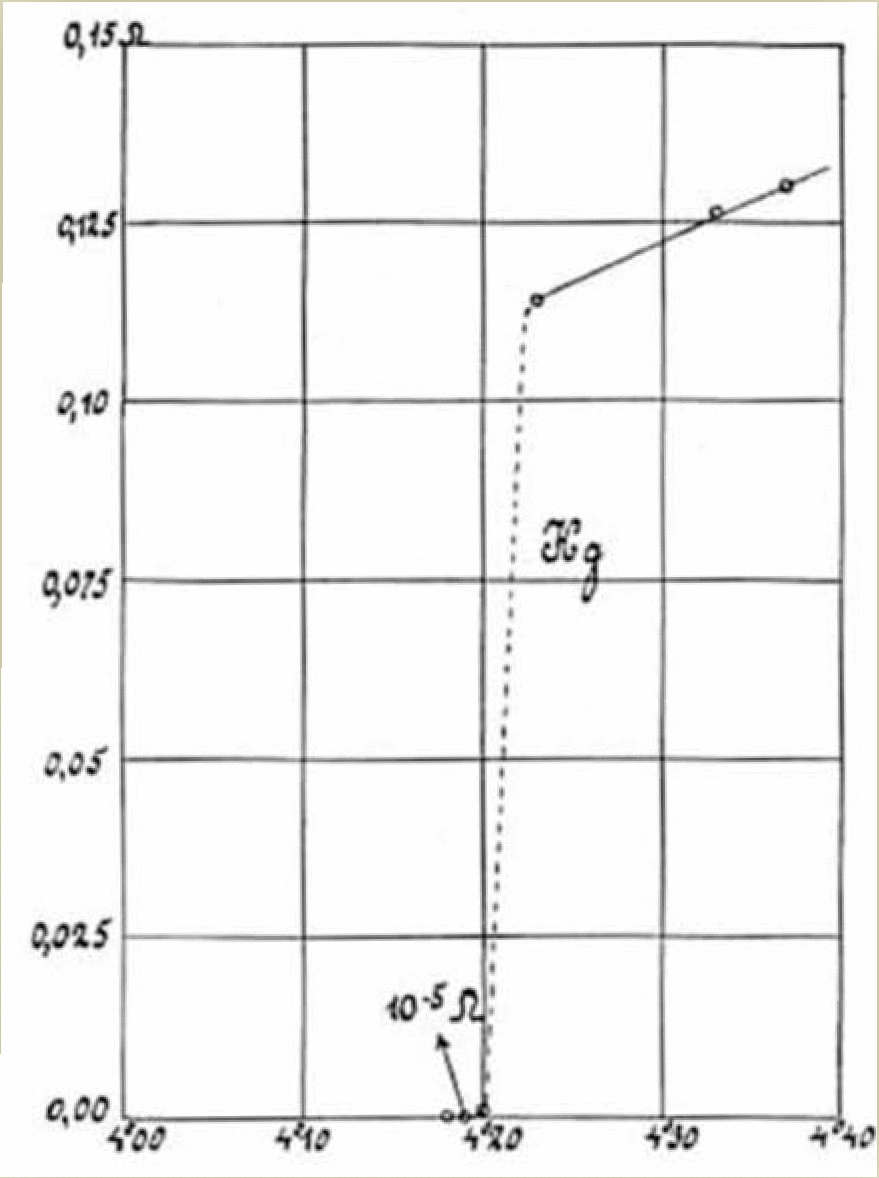
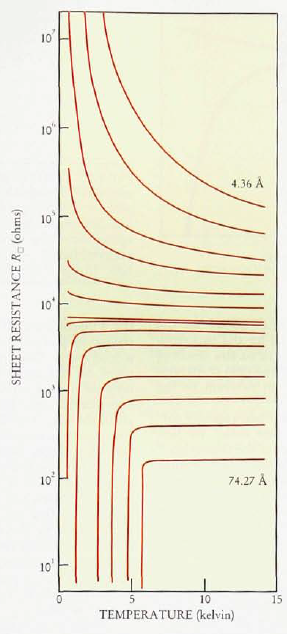
31. AMORPHOUS BISMUTH is wild because it shows relatively robust superconductivity despite having no microscopic order. Most dramatic of all, though, is that it shows a direct transition from superconducting (zero resistance) to insulating (infinite resistance) states.
This graph announced the discovery, and is still one of the most dramatic graphs I have seen.
(Different curves correspond to different thicknesses of the material).
(Different curves correspond to different thicknesses of the material).
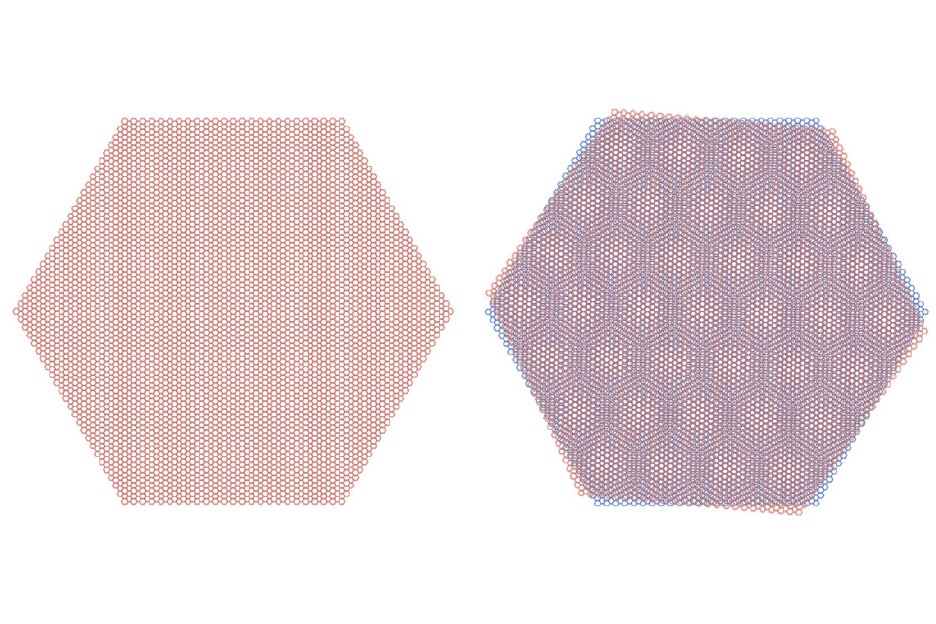
32. MAGIC ANGLE GRAPHENE is a very recent and shocking discovery (that happened down the hall from me while I was at MIT). All you do is take two pieces of graphene and twist them slightly with respect to each other, which forms a moiré pattern.
For some (still unknown) reason this new twisted material acts like a cuprate superconductor! Even though neither graphene sheet by itself has any hints of superconductivity (or insulating behavior).
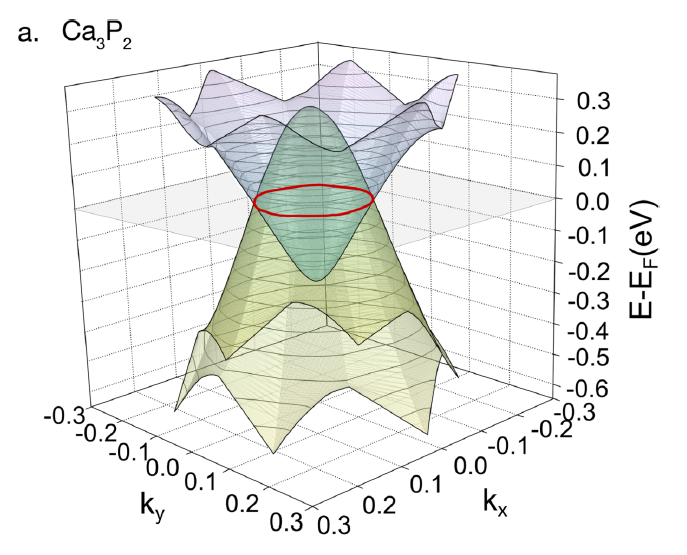
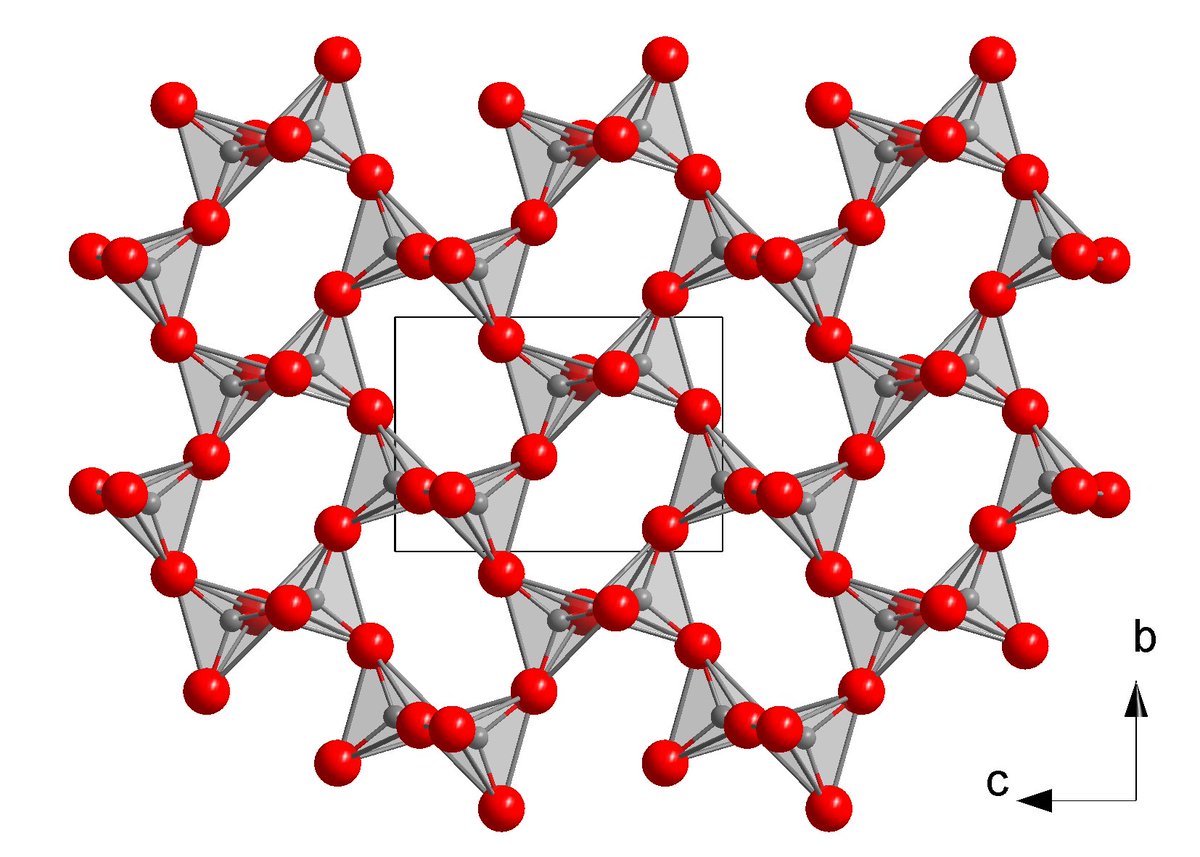
33. CALCIUM DIPHOSPHIDE has a recently-discovered crystal form (first synthesized by @lilia_xie as an undergrad). It's what's called a "nodal line semimetal": an electron with zero energy does not have zero momentum p, but has any value of p that resides along a ring-shaped space
A wild thing about Ca3P2 is that electric charges inside it do not have the usual 1/r^2 force law. Instead, the force goes like 1/r^3 !
This change comes from a strong effect of virtual "particle-antiparticle" pairs, which briefly appear from the ground state for quantum reasons.
This change comes from a strong effect of virtual "particle-antiparticle" pairs, which briefly appear from the ground state for quantum reasons.
34. CALCITE is a material that exhibits birefringence, which means that it has two different indices of refraction. Which way light bends when going through calcite depends on the light's polarization (the direction of its electric field).
So normal unpolarized light goes two directions at once, leading to a doubling of images:
35. A LIQUID CRYSTAL is a material made up of long molecules that flow like a liquid, but are locally aligned in an orderly way, like a crystal. They have a million different phases, each with subtly different properties.
One cool thing about liquid crystals is that they typically act like polarizers (pass light only when its electric field points in a particular direction). They can also be aligned with a static voltage. This effect is what enables LCD technology.
36. In lieu of telling you anything cool about GLASS, I'll refer you to this excellent thread: https://twitter.com/VitreousSolid/status/1339347539200503813
(Okay, I'll tell you one thing about glass. Any time someone says "actually, glass is just a liquid that flows extremely slow", a materials scientist somewhere gets pissed off.)
("No, really! That's why the glass windows in really old churches look so wavy!")
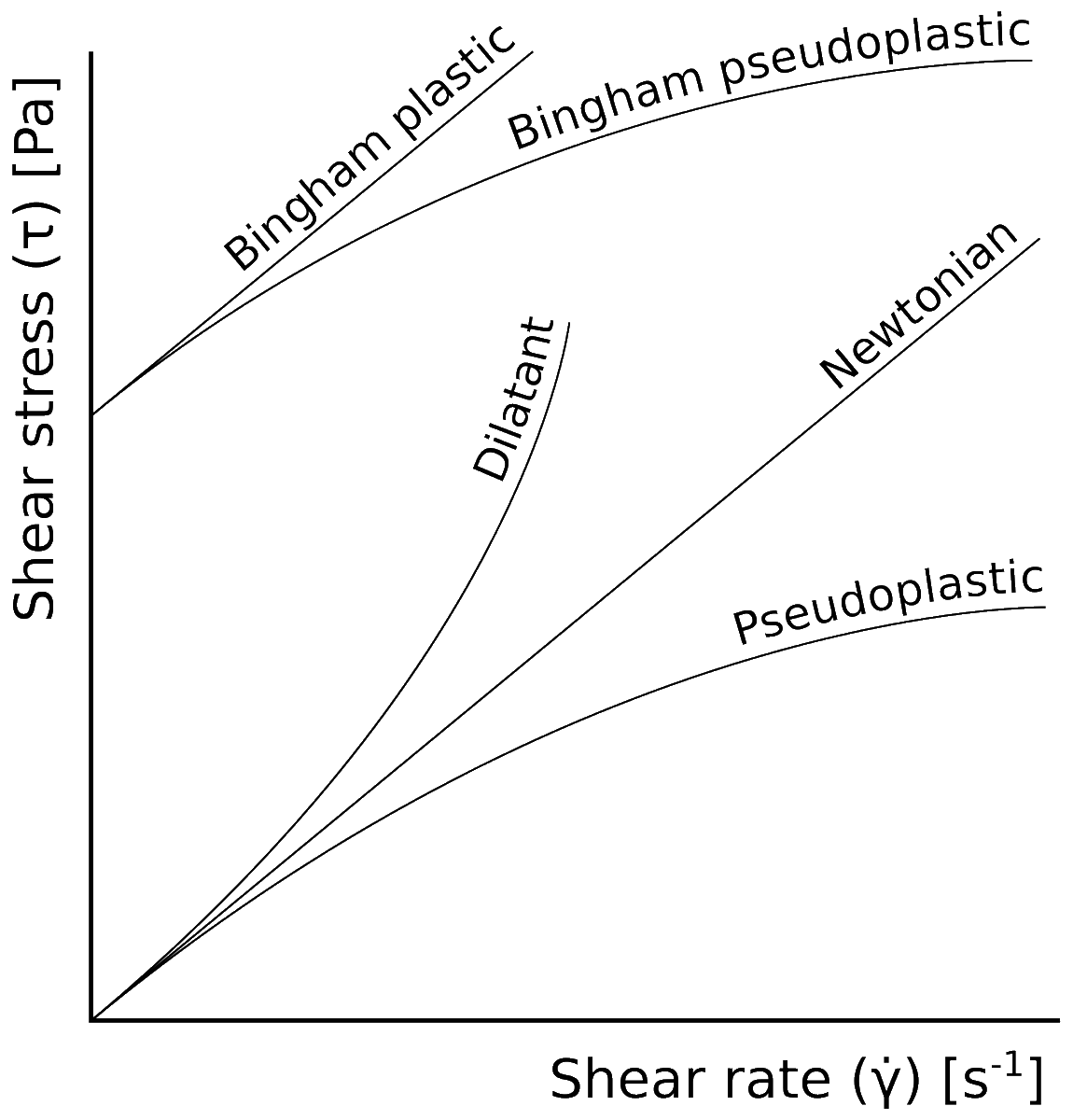
37. A NON-NEWTONIAN FLUID (like cornstarch in water) is one for which the apparent viscosity depends very strongly on the shear rate. There are all sorts of crazy demos of these online.
But the truth is that there is no sharp distinction between a non-Newtonian fluid as compared to a "normal" fluid. It just happens that the usual Taylor expansion of stress vs. strain rate breaks down really quickly.
(Now thinking about having a day in Calculus class where you force students to punch a block of cornstarch water, and then say:
"You see! This is what happens when you rely too heavily on the first-order Taylor expansion!!")
"You see! This is what happens when you rely too heavily on the first-order Taylor expansion!!")
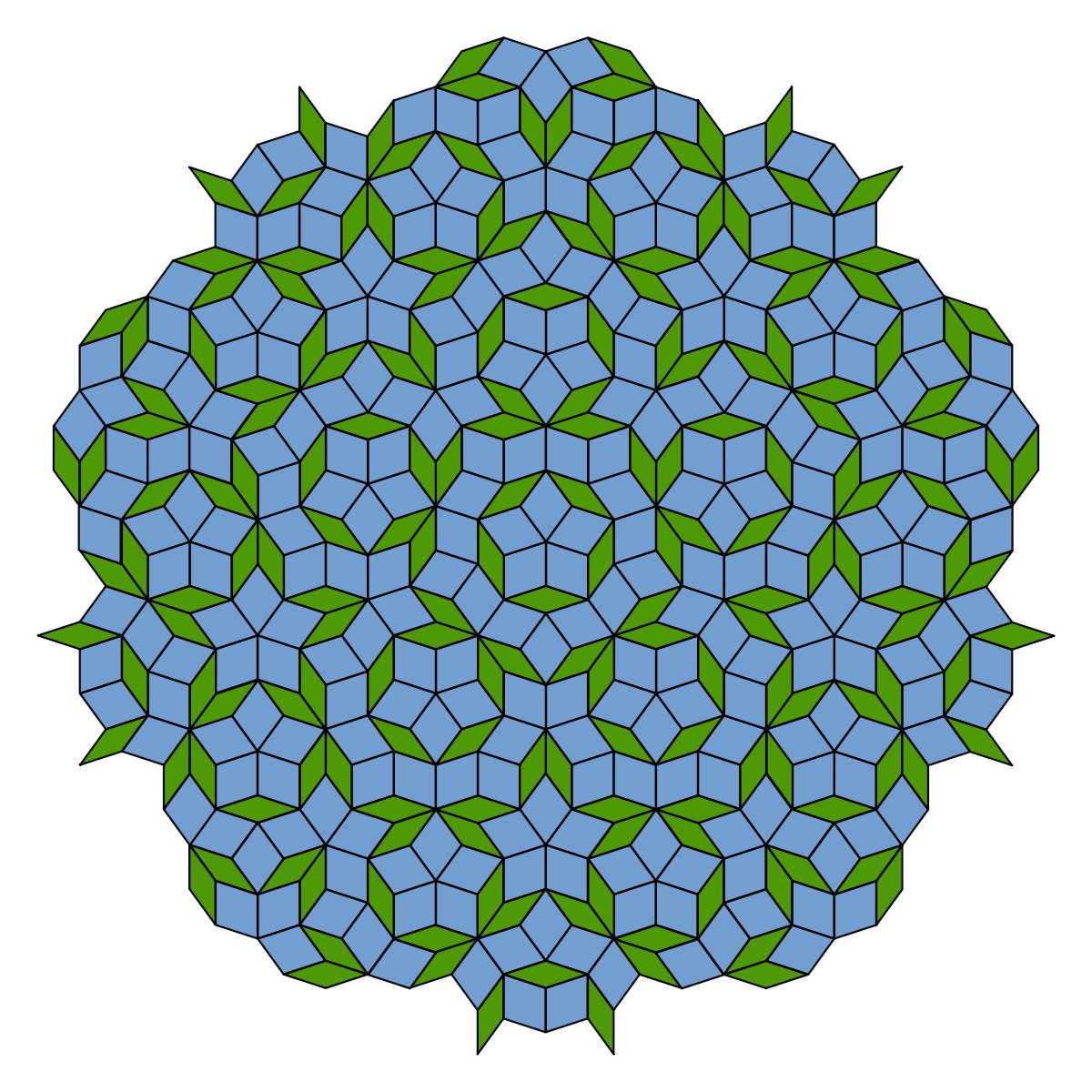
38. QUASICRYSTALS are materials for which the symmetry is inconsistent with a periodic tiling. For example, a five-fold symmetric crystal is not supposed to exist, since it's impossible to tile space with pentagons.
Nonetheless, such materials can exist!
Nonetheless, such materials can exist!
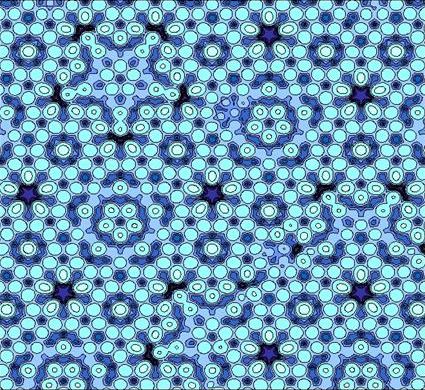
They just have tiling that is locally regular but has no long-range order, like Penrose tiles.
(left: a real microscopy image. right: Penrose tiles)
(left: a real microscopy image. right: Penrose tiles)
All the quasicrystals we have found on earth are apparently meteorite fragments.
If you ever want to hear an incredible, adventurous science talk, listen to Paul Steinhardt talk about leading an expedition into Kamchatka to find them:
If you ever want to hear an incredible, adventurous science talk, listen to Paul Steinhardt talk about leading an expedition into Kamchatka to find them:
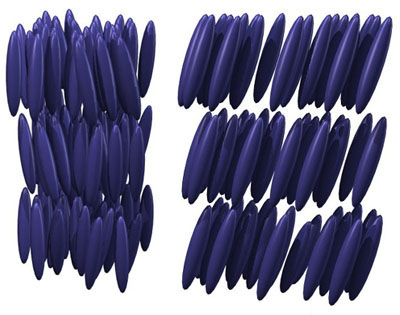
39. Most materials expand in one direction when you compress them in a perpendicular direction. But AUXETICS are materials that either shrink or expand in both directions simultaneously.
(In engineering terms, they have a negative "Poisson ratio")
(In engineering terms, they have a negative "Poisson ratio")
There are lots of fun engineering examples of auxetics. But they also appear naturally. Here's one, for example ("cristobalite", a variant of quartz):
40. OPTICAL METAMATERIALS are human-made materials in which a bunch of small electromagnetic resonators are arranged so that their interactions produce unusual behaviors of light (or EM waves in some band of frequency). For example, you can engineer negative index of refraction!
41. There is a lot of excitement and mystery right now about SAMARIUM HEXABORIDE, which looks simultaneously like an insulator and a quantum metal, in ways that we are still grappling with.
See this earlier thread: ( https://twitter.com/gravity_levity/status/1179469564423225344)
See this earlier thread: ( https://twitter.com/gravity_levity/status/1179469564423225344)
I think that I am out of time and energy now, and tired of spamming your timelines. So let's draw this thread to an
END.
Thank you all for coming along with me. It's been fun, and nothing at all like a systematic list of "the 100 most interesting materials".
END.
Thank you all for coming along with me. It's been fun, and nothing at all like a systematic list of "the 100 most interesting materials".
@vgr I didn't get to 100, but this thread has at least 100 tweets in it and I am now reporting for debriefing

 Read on Twitter
Read on Twitter