How does neural connectivity support learning and memory? This (very long) paper provides insights, diving deep into the connectome of the fly mushroom body. A huge team effort that I was fortunate to be part of. Here are some of my favorite parts (1/22) https://elifesciences.org/articles/62576
Disclaimer: this isn’t a paper that can be summarized on Twitter. Instead, I’ll briefly point out some high-level ideas that might especially interest a computational neuro audience. Those with different backgrounds might have completely different takeaways! (2/22)
Background: The mushroom body (MB) processes sensory info w/ a largely feedforward structure: projection neurons (PNs) -> Kenyon Cells (KCs, high-dim + sparse) -> output neurons (MBONs). MBONs are organized in compartments innervated by dopamine neurons (DANs). (3/22)
KC->MBON synapses undergo plasticity when KCs and corresponding DANs are co-active (in a timing-dependent fashion). This plasticity is known to underlie classical associative learning in the fly (e.g. odor-reward pairings). (4/22)
Why study the MB? Aside from flies being cool, it is a tractable and well-characterized model system for learning/plasticity with implications for many other species / systems. (5/22)
The MB described as “cerebellum-like,” with DAN-driven plasticity analogous to complex spike-driven plasticity. The emerging role of plateau potentials / burst firing in hippocampal and cortical plasticity suggests related mechanisms may be at play in those areas as well. (6/22)
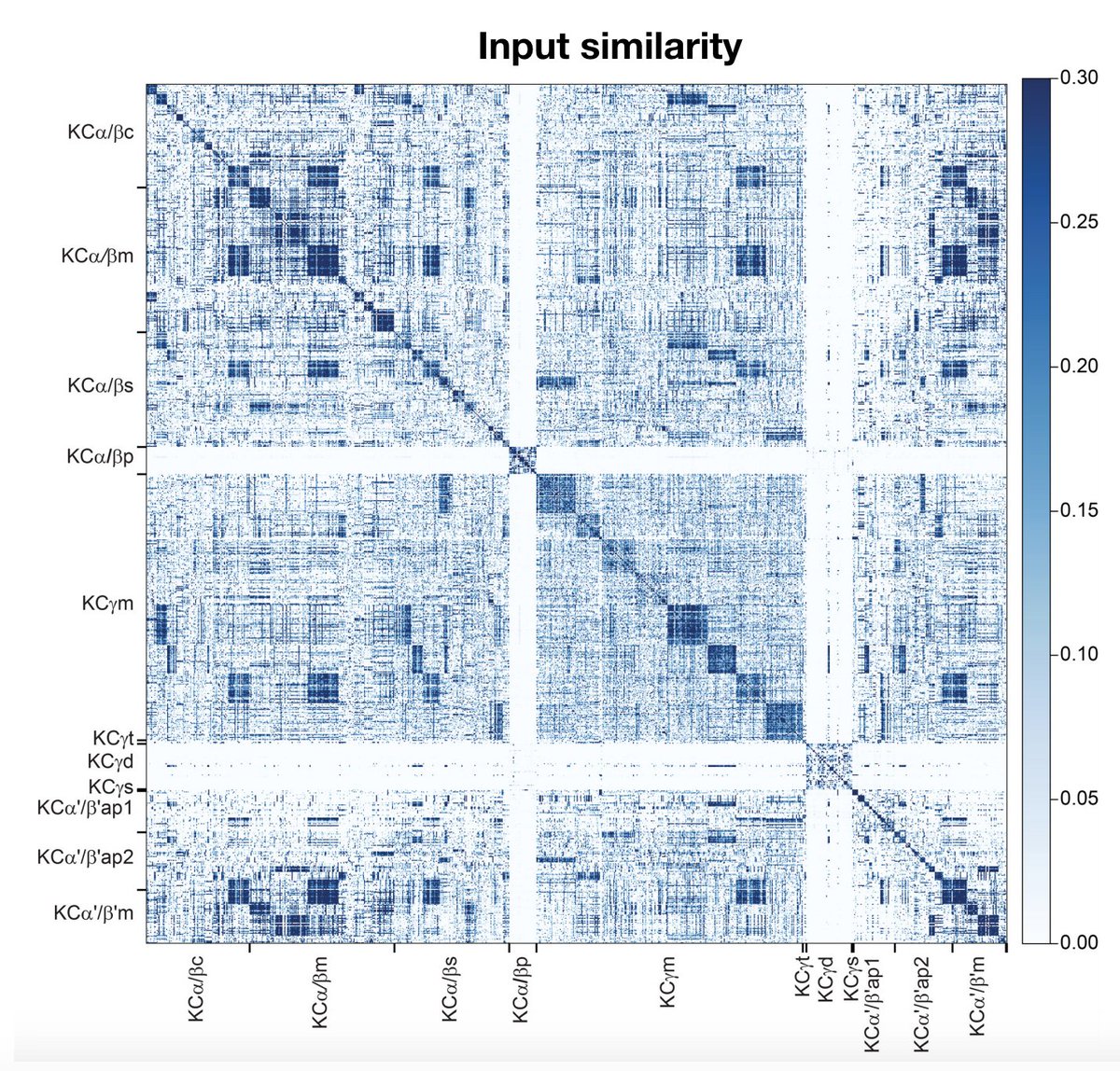
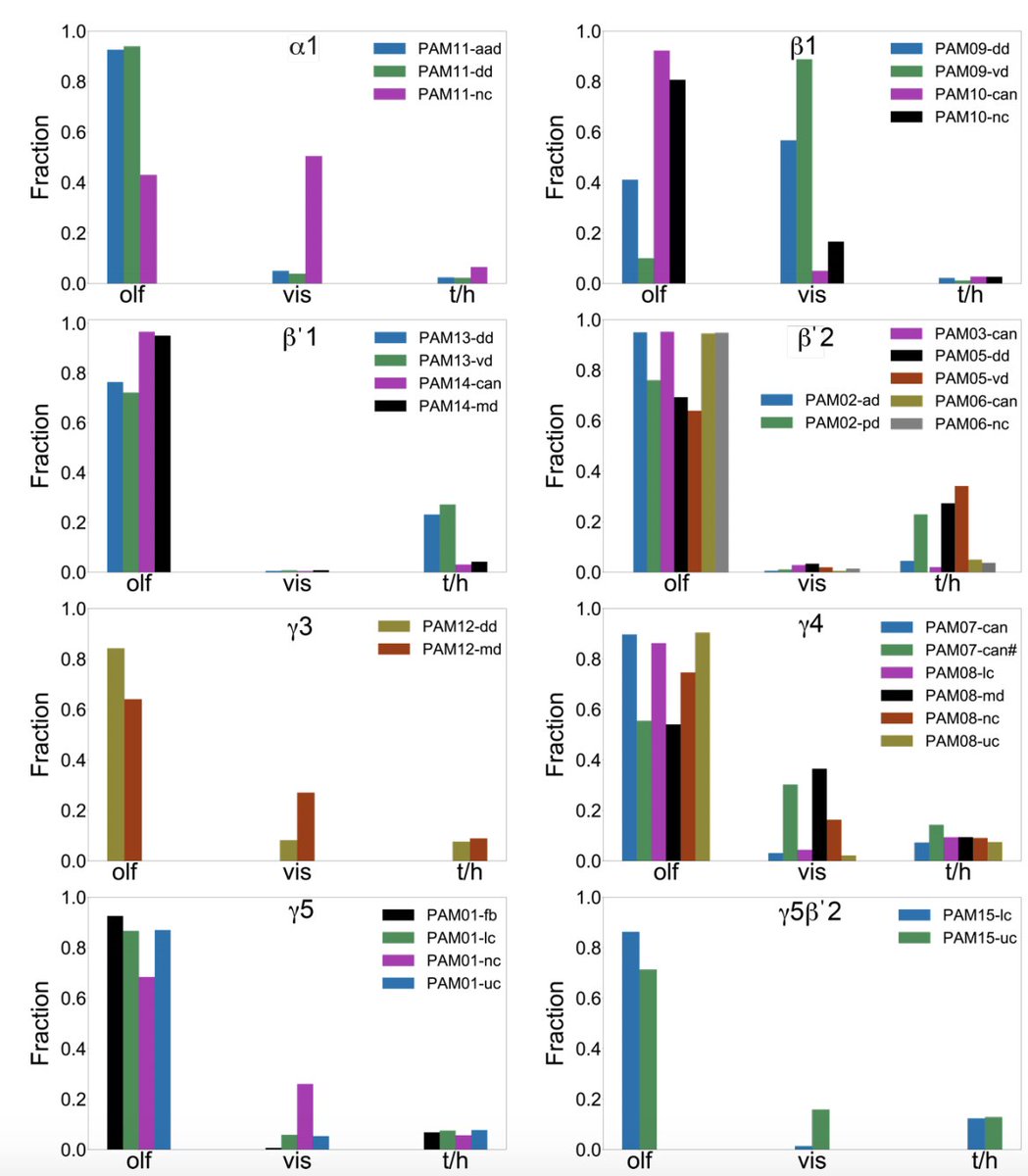
Highlight #1: PN->KC connectivity has previously been modeled as random, an organization which (via expansion of representation dimensionality) supports discrimination between many sensory stimuli. The data, however, reveals significant deviations from randomness… (7/22)
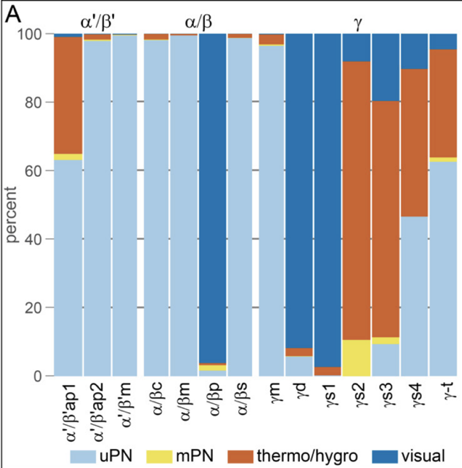
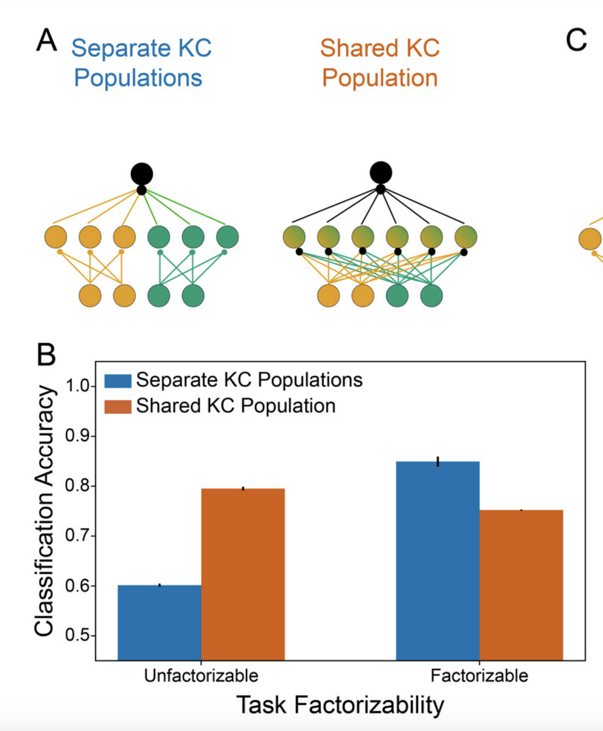
…most strikingly at the level of KC subtypes specializing for different sensory modalities. We suggest this architecture reflects a prior that the association between multimodal stimuli and their valences is factorizable across modalities (no olfactory-visual XORs). (8/22)
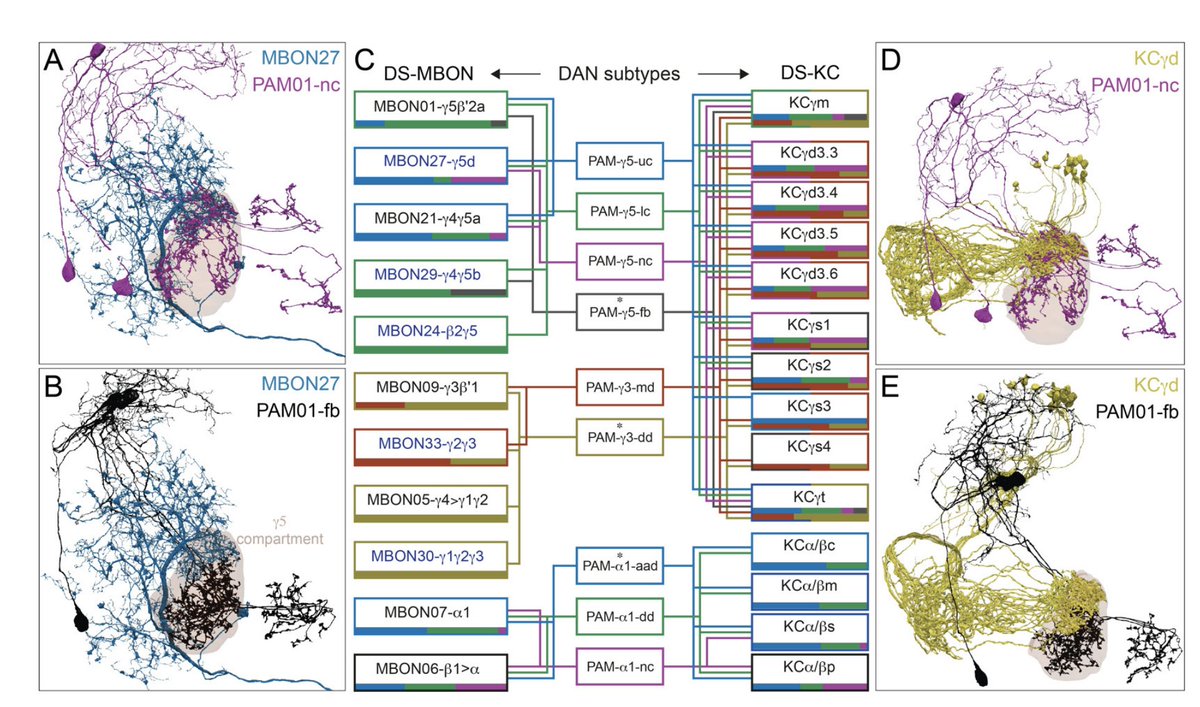
Highlight #2: The connectome data reveal unexpected subcompartmental organization of DAN axon targets. Different DAN subtypes exhibit preferences for different KC subtypes, potentially enabling (for example) modality-specific dopaminergic learning signals. (9/22)
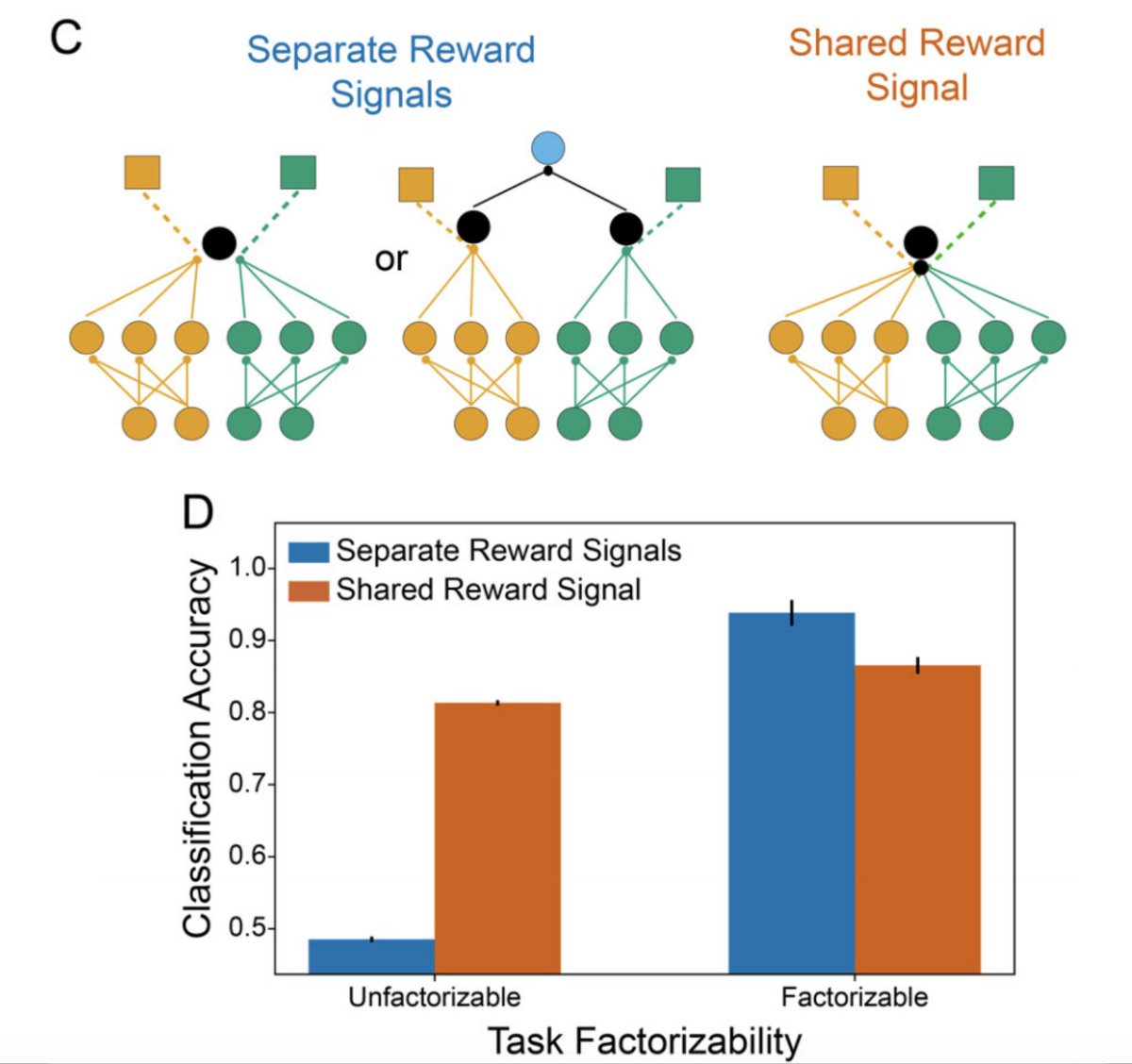
Mathy interpretation: this organization allows high-rank updates to the KC-MBON weight matrix, rather than the rank-1 updates given by Hebbian-style (or ML-style) learning rules. Modeling suggests that this flexibility can be beneficial given a factorizable task structure (10/22)
Highlight #3: We investigated structure in the inputs to DANs. We found substantial heterogeneity in their inputs (40 putative functional groups, consistent with morphology), which could support high-dimensional learning signals (as opposed to scalar reward/punishment) (11/22)
Also, connectivity-based clusters include DANs from different compartments, suggesting that such signals may be distributed across the population. These findings motivate extensions to classical RL models that incorporate distributed, vector-valued learning signals (12/22)
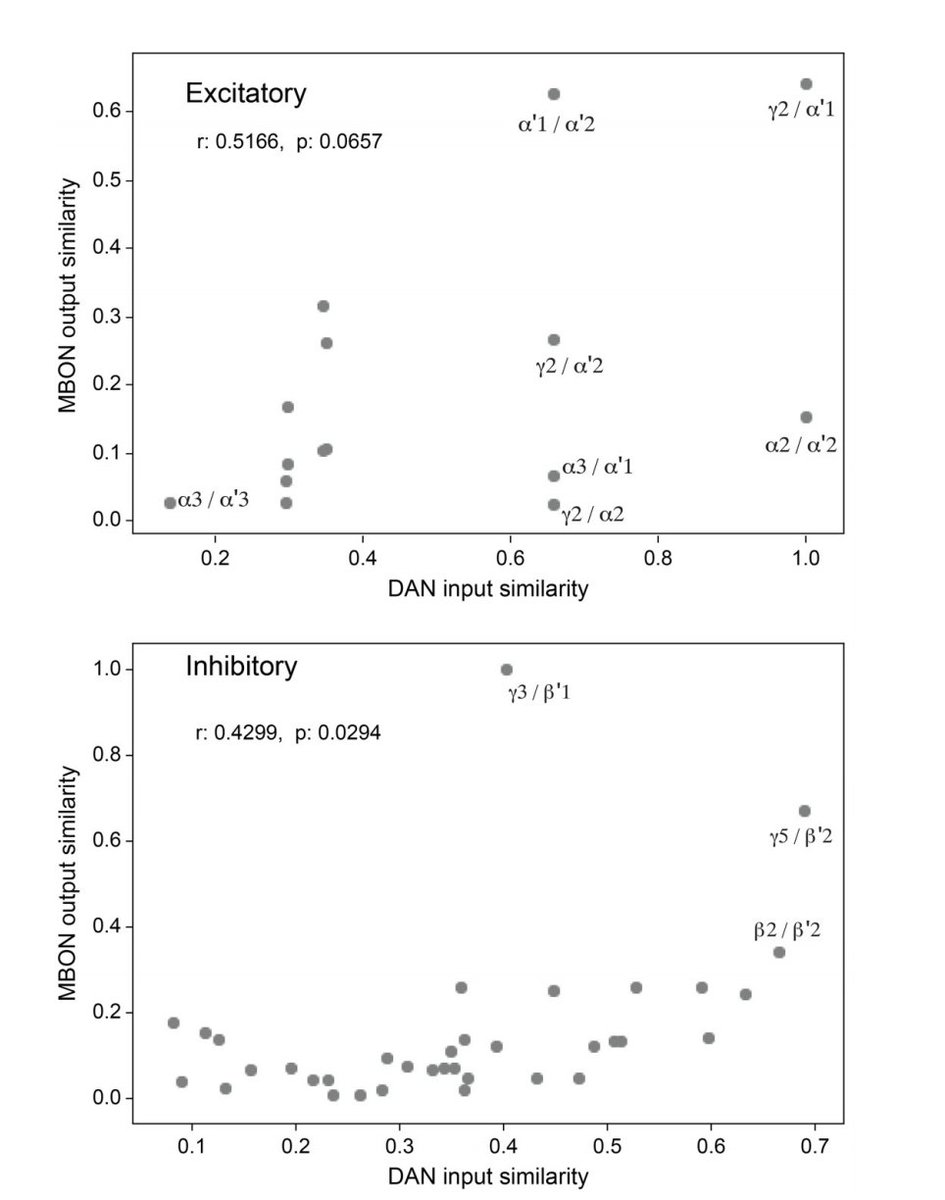
To some extent, similarity of inputs to DANs correlates with similarity of outputs from their corresponding MBONs, perhaps reflecting a form of credit assignment in which compartments receive different learning signals dependent on their functional/behavioral roles (13/22)
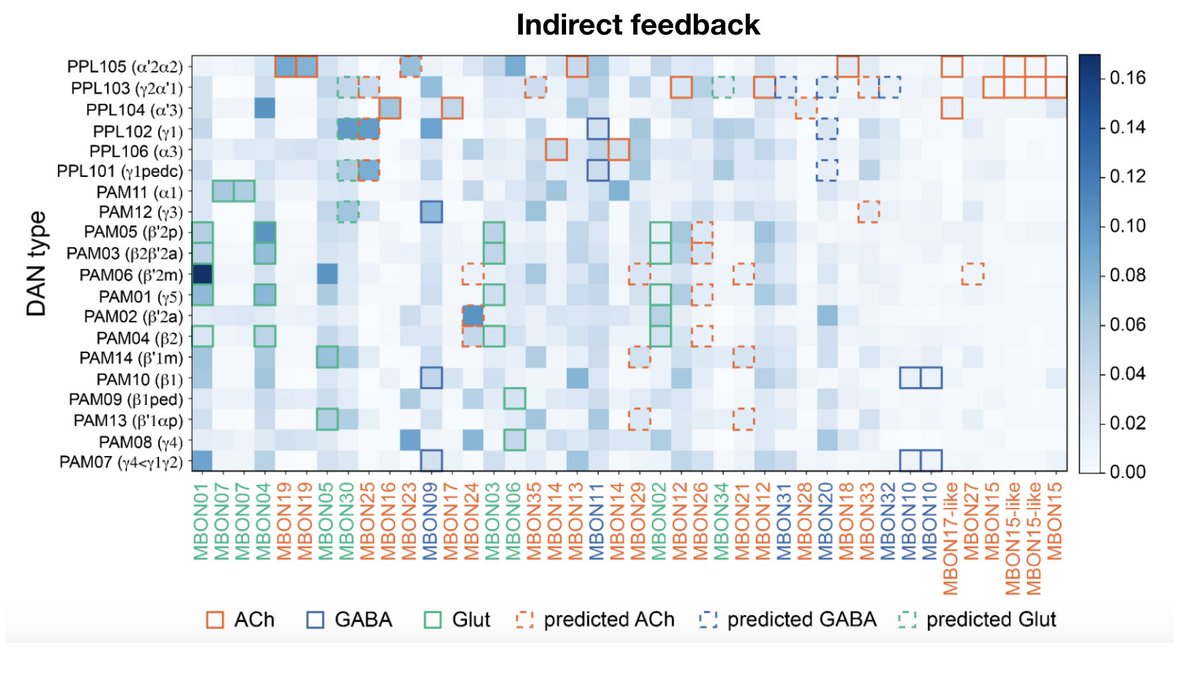
Highlight #4: DANs receive extensive modulation from MBONs, both direct and multisynaptic, within and across compartments. Broadly, this suggests a “critic” role for MBONs in contributing to learning signals, beyond their classical “actor” role in driving behaviors. (14/22)
Cross-compartment feedback can also gate the learning/consolidation of certain associations contingent on others already having been learned, a phenomenon observed in short->long-term memory transfer. Currently working on modeling this sort of mechanism, stay tuned! (15/22)
Interesting patterns can be found in the MBON-DAN connectivity. For instance, glutamatergic MBONs appear particularly biased toward same-compartment feedback, both direct and indirect. (16/22)
Highlight #5: MBONs interact with one another in an extensive, largely hierarchical/feedforward network (w/ axo-dendritic and axo-axonal connections). This enables e.g. gating of certain MBONs by others that can be released through disinhibition following learning. (17/22)
This architecture challenges the conception of MBONs as pure “output” units and KC-MBON plasticity as “fitting readout weights" -- rather, this plasticity is upstream of much layered, nonlinear computation (to ponder: are there computational benefits to this arrangement?) (18/22)
Highlight #6: The outputs from MBONs are heterogeneous but reveal some organizational principles. MBONs with overlapping sources of sensory inputs tend to have (but don't always) have more convergent outputs, suggesting parallel sensory->behavior processing pathways. (19/22)
Another observation is the presence of pairs of cholinergic / glutamatergic MBONs converging on common targets -- these may exert competing excitatory / inhibitory influences, consistent with their previously described role in driving avoidance / approach, respectively (20/22)
To reiterate, these tweets cover only a small and biased subsample of the paper. Read it for the full scoop! Many thanks to Feng Li and Gerry Rubin for leading this effort, Ashok Litwin-Kumar and Larry Abbott for advising / helping with my contributions, … (21/22)
and @_Nils_Otto_, Lisa Marin, @gsxej, @ScottishWaddell, and many other wonderful collaborators for being great to work with + getting me up to speed (at least a little) on fly neuroscience, and of course @janeliaflyEM for this awesome data. (22/22)

 Read on Twitter
Read on Twitter