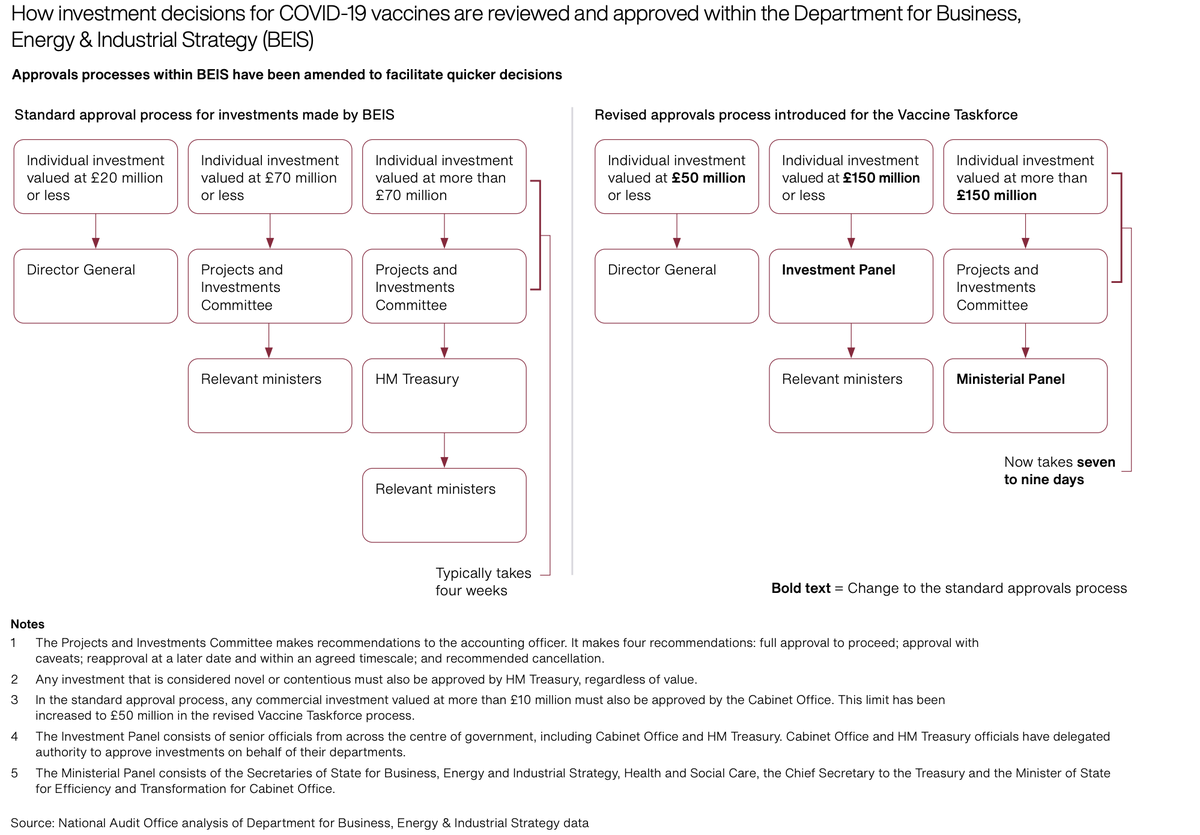
UK NAO watchdog on vaccine spend: over £11bn committed; indemnity protection for pharma
companies in event of liabilities/legal action due to adverse effects: in 4 out of five contracts
agreed, no cap applied to the amount taxpayers could
pay... https://www.nao.org.uk/wp-content/uploads/2020/12/Investigation-into-preparations-for-potential-COVID-19-vaccines.pdf
companies in event of liabilities/legal action due to adverse effects: in 4 out of five contracts
agreed, no cap applied to the amount taxpayers could
pay... https://www.nao.org.uk/wp-content/uploads/2020/12/Investigation-into-preparations-for-potential-COVID-19-vaccines.pdf
Indemnity protection is not just about taxpayers footing lawsuit bill; coupled with fast track approvals and large pay outs de-liked from performance, it is also a disincentive for companies to complete trials + proactively report/protect against any side effects of their product
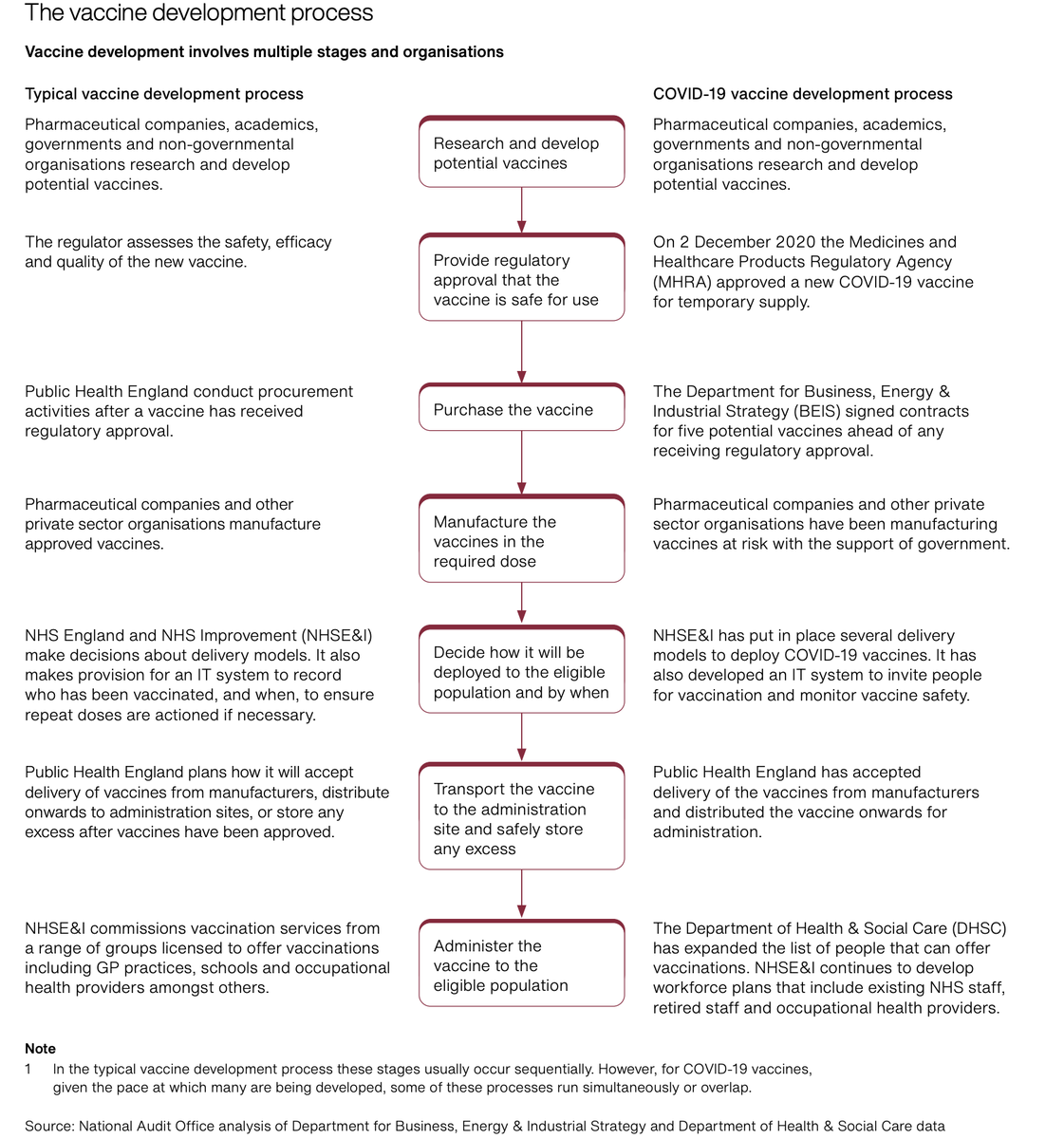
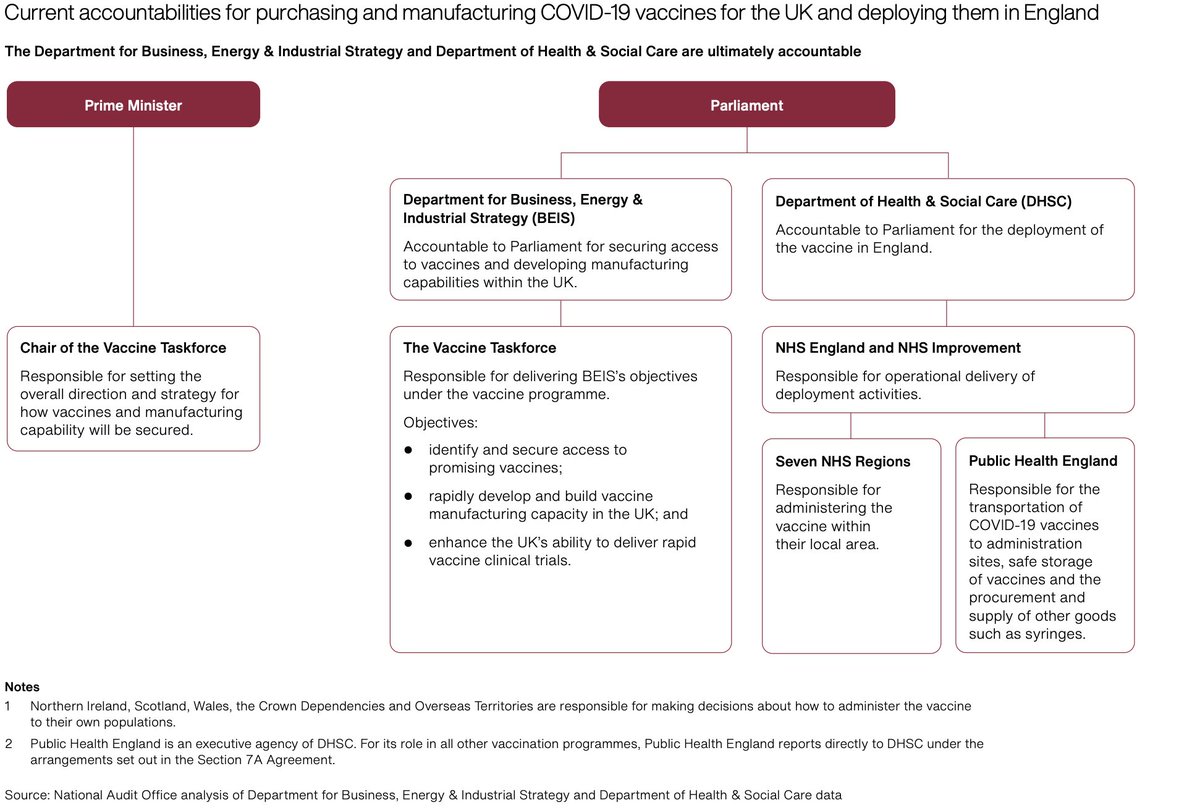
Startling degree of complexity in decision making and planning from product selection to procurement to deployment + public comms highlights challenges for less well resourced countries despite WHO/Covax; uphill struggle. Distribution costs almost as high as acquisition...In UK!
"NHS calculated it may need up to 46,000 staff incl 26,000 vaccinators and 20,000 administrative staff based on 75% take-up rate...recruitment of staff taking place when there are workforce shortages and concerns about the well-being of existing staff due to the pandemic"
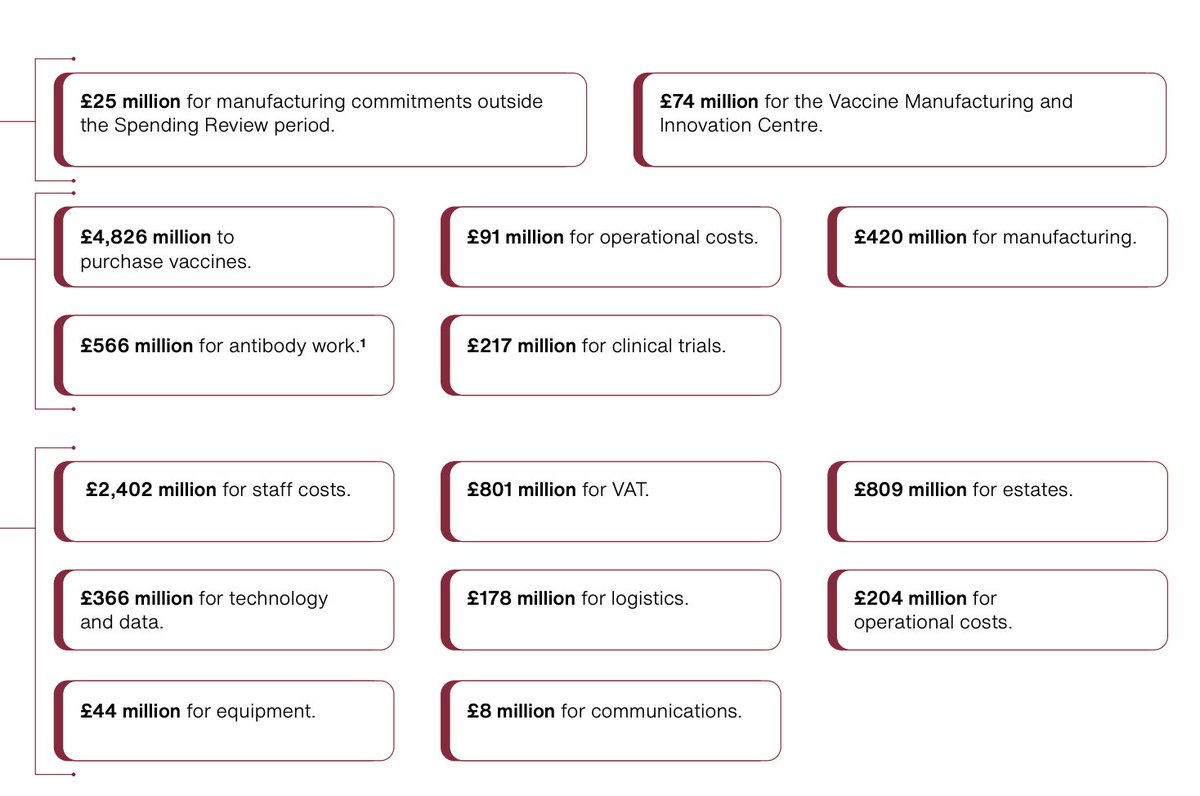
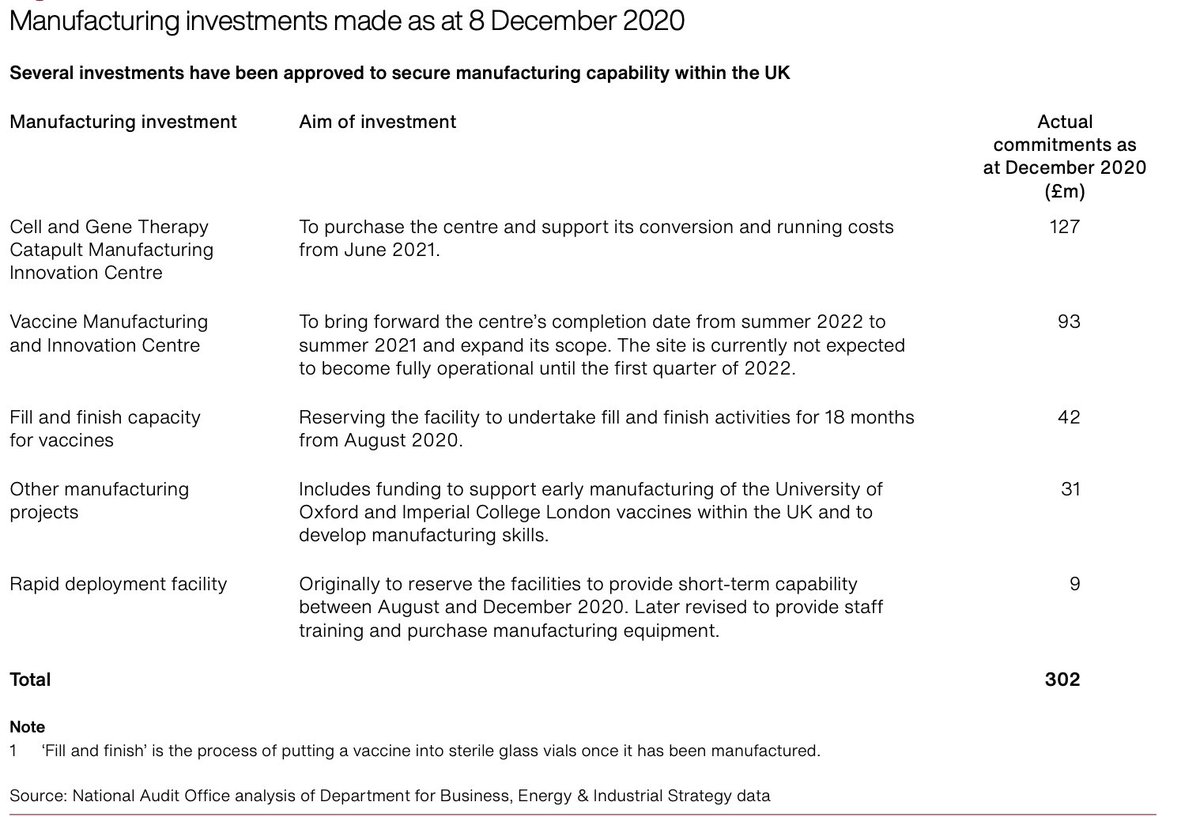
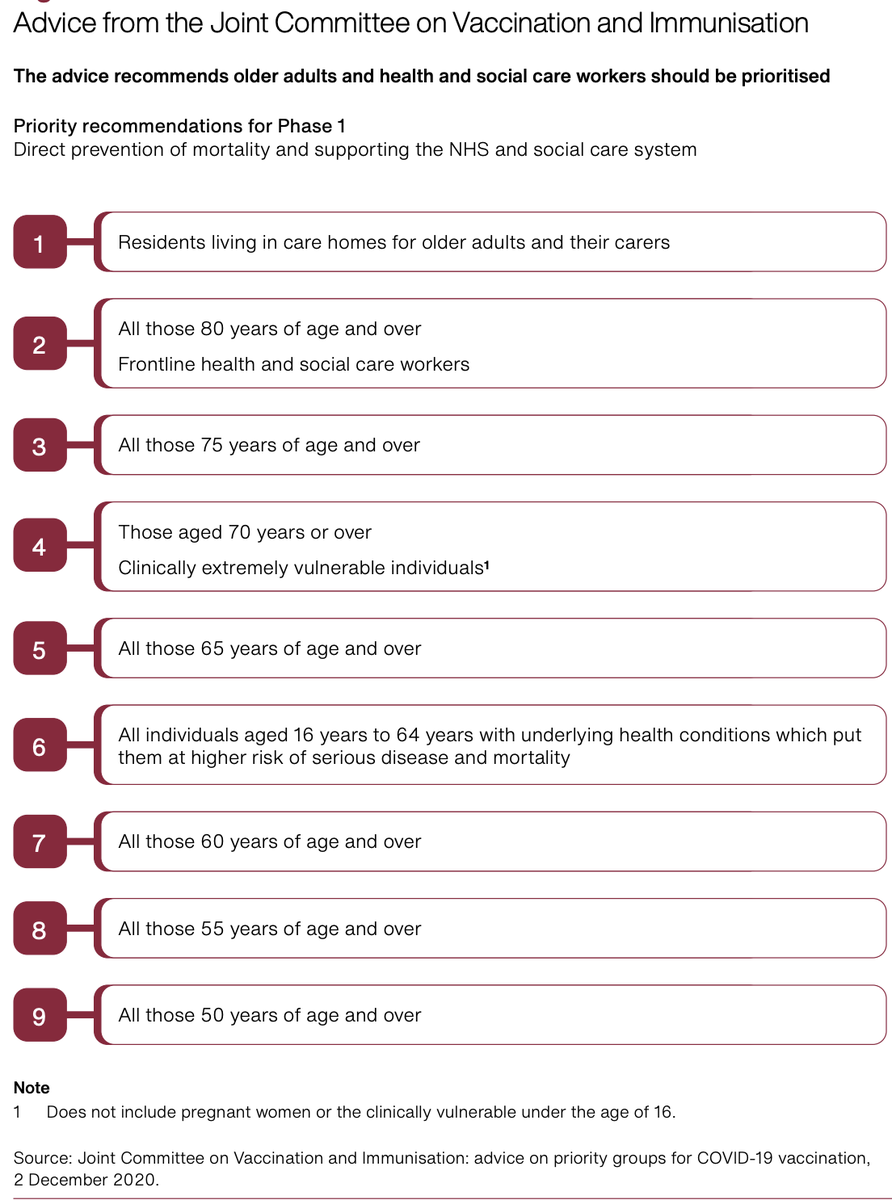
This is the manufacturing investment of the UK broken down and three roll out modalities and prioritisation criteria (age)

 Read on Twitter
Read on Twitter