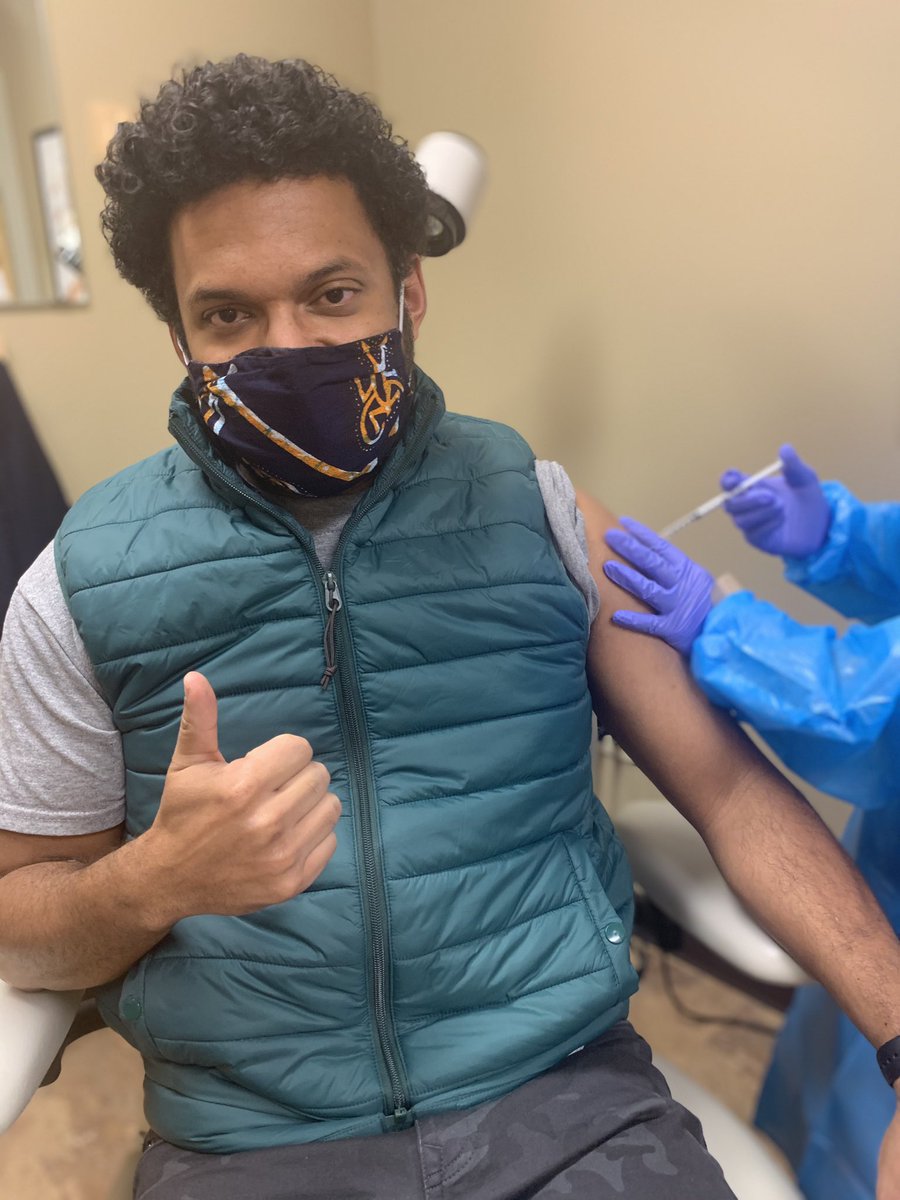
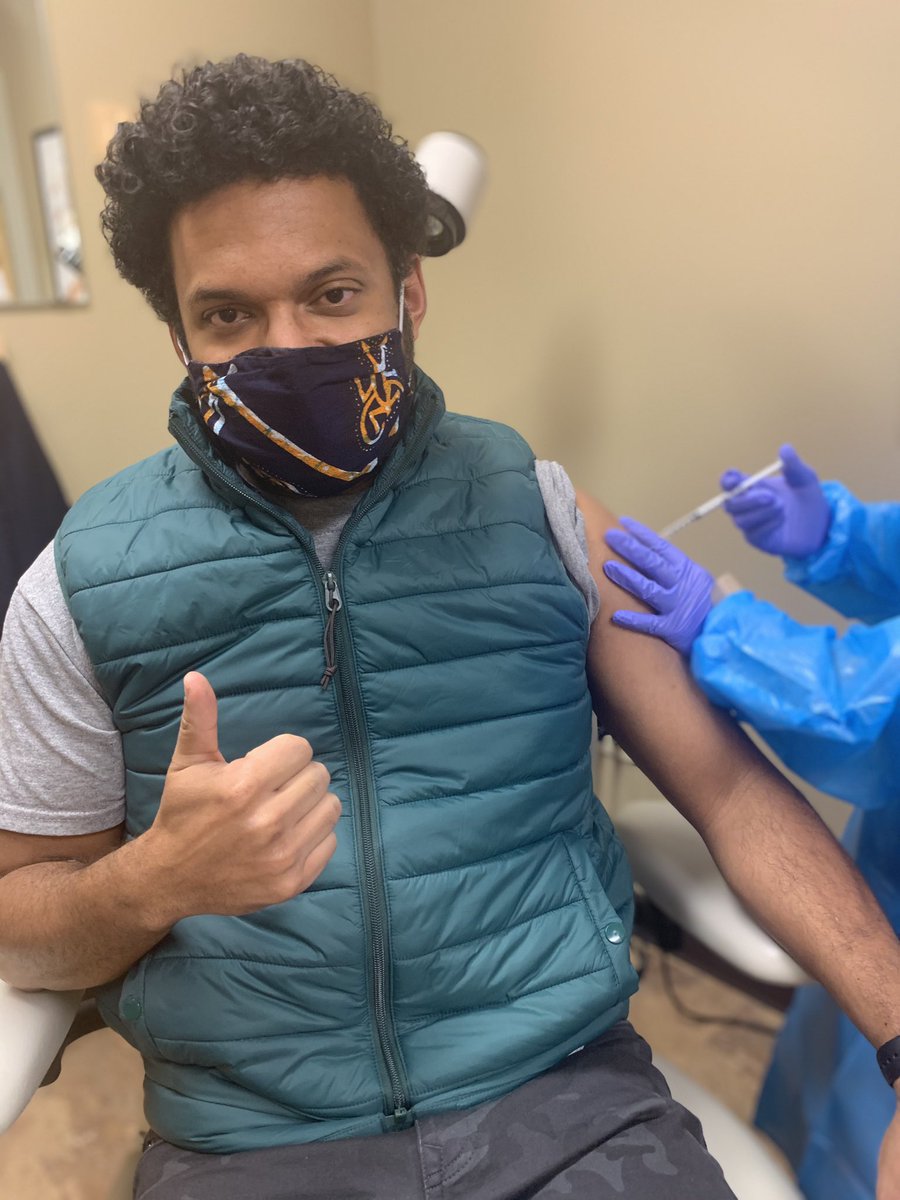
I have enrolled in a #COVIDvaccine trial & last week got my first shot!!
But this wasn’t always my plan. I had a surprising amount of hesitancy early on. So I want to share how I worked through it.
This will probably be my longest thread ever. But stick w/me! (1/)
But this wasn’t always my plan. I had a surprising amount of hesitancy early on. So I want to share how I worked through it.
This will probably be my longest thread ever. But stick w/me! (1/)
An avid advocate that #vaccinessavelives I found myself personally & professionally skeptical about the speed with which vaccines were being developed & politicized.
So I read about #COVIDvaccine development & listened to @NEJM’s podcast weekly https://podcasts.apple.com/us/podcast/new-england-journal-of-medicine-interviews/id207118381?i=1000472332919 (2/)
So I read about #COVIDvaccine development & listened to @NEJM’s podcast weekly https://podcasts.apple.com/us/podcast/new-england-journal-of-medicine-interviews/id207118381?i=1000472332919 (2/)
I also found this @nytimes #COVID19 vaccine tracker helpful
https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
(3/)
https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
(3/)
Additionally, in talking to Infectious Disease colleagues of mine previous vaccine trials for SARS-CoV & MERS-CoV (Covid’s “cousins”) were referenced as a foundation for the science behind many #Covid19 vaccine trials. Here is a nice review of that:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7177048/
(4/)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7177048/
(4/)
Following these resources helped me overcome my initial hesitancy based on the speed w/which these vaccines have been developed.
Ive shared my path of gathering info w/my patients/parents & lately Ive been using this analogy (s/o @Theresa_Chapple ) https://twitter.com/theresa_chapple/status/1333742074496225281?s=21
) https://twitter.com/theresa_chapple/status/1333742074496225281?s=21
Ive shared my path of gathering info w/my patients/parents & lately Ive been using this analogy (s/o @Theresa_Chapple
 ) https://twitter.com/theresa_chapple/status/1333742074496225281?s=21
) https://twitter.com/theresa_chapple/status/1333742074496225281?s=21
So next was concern about the mRNA vaccine specifically, as it has not yet been rolled out to millions of individuals.
Learning more about the history of #mRNA technology & the companies utilizing it has helped. This is a particularly good piece: https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/
(6/)
Learning more about the history of #mRNA technology & the companies utilizing it has helped. This is a particularly good piece: https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/
(6/)
However we still don’t know what the possible short/medium/long term side effects, although likely rare, could be when this is administered to millions.
That said, vaccines have always been about decreasing risk & #COVID19 presents a significant risk to many of our communities.
That said, vaccines have always been about decreasing risk & #COVID19 presents a significant risk to many of our communities.
Additionally, most proven vaccine-related long term side effects have shown up within months, not years.
So I am reassured that we haven’t yet seen any significant number of side effects in thousands of participants who received their vaccine 4+ months ago
(8/)
So I am reassured that we haven’t yet seen any significant number of side effects in thousands of participants who received their vaccine 4+ months ago
(8/)
Given the risk of getting #COVID19 & what we don’t know about its longterm effects; when family/friends/patients ask about mRNA vaccines, my response is:
“the unknown short/long term risk of a mRNA vaccine is still much more acceptable to me than the risk of getting COVID”
(9/)
“the unknown short/long term risk of a mRNA vaccine is still much more acceptable to me than the risk of getting COVID”
(9/)
Once ready to get my vaccine I had a decision: wait for an early approved vaccine as a health care worker likely to get early priority?
Or participate in a #CovidVaccine trial?
Or participate in a #CovidVaccine trial?
In the Twin Cities, my employer was the 1st to begin enrolling participants in a #COVID vaccine trial. You may have heard of the AstraZeneca/Oxford trial that had to be paused. That is the one.
The other current option in the Twin Cities is with Janssen Pharmaceuticals.
(11/)
The other current option in the Twin Cities is with Janssen Pharmaceuticals.
(11/)
Both use a more “traditional” approach of a genetically modified virus to teach the immune system how to make a protective response against SARS-CoV-2.
In addition to the global pause the AZ/Oxford trial also realized there had been a mistake in some of the initial doses with some participants getting a 1/2 dose.
Thing is those participants actually had a better response.
Yet it remains that a miscalculation was made.
(13/)
Thing is those participants actually had a better response.
Yet it remains that a miscalculation was made.
(13/)
So to some the answer may seem obvious, go w/the trial that hasn’t had to be halted or had a mistake, right?
But yet, to me, those steps means those running the trial aren’t cutting corners & are willing to admit missteps.
Appropriate transparency is paramount. You know why.
But yet, to me, those steps means those running the trial aren’t cutting corners & are willing to admit missteps.
Appropriate transparency is paramount. You know why.
Here’s where the AZ/Oxford trial has 1 thing in MN that the Jansssen trial doesn’t - @ZekeMD
Zeke & I go back to medical school where we worked together @SNMA helping fill our fellow students’ gaps in education around #SDOH before it became a more regular part of the curriculum
Zeke & I go back to medical school where we worked together @SNMA helping fill our fellow students’ gaps in education around #SDOH before it became a more regular part of the curriculum
. @ZekeMD continues that education today as chief medical editor of @mnmed’s MN Medicine, member of @MNAADocs & is a lead investigator of the MN Oxford/AZ trial
He spoke about how #COVID19 vaccine trials help protect human rights in @MNSpokesman
(16/) https://spokesman-recorder.com/2020/12/05/covid-19-trials-protect-human-rights/

He spoke about how #COVID19 vaccine trials help protect human rights in @MNSpokesman
(16/) https://spokesman-recorder.com/2020/12/05/covid-19-trials-protect-human-rights/
Although most of my time, focus & energy has been on #COVID19 here in the US, as someone w/family around the world, & particularly in Cameroon, I also wanted to support a vaccine that could truly be global.
Ultra low freezers & cold chains aren’t going to work in much of Africa.
Ultra low freezers & cold chains aren’t going to work in much of Africa.
Knowing the Oxford scientists used their leverage to get AZ on board with making their #COVIDvaccine on a not-for-profit basis worldwide, for the duration of the pandemic, & always at cost to low- & middle-income countries, was also a critical factor
#HealthIsAHumanRight
(18/)

#HealthIsAHumanRight
(18/)
This recently published piece does a great job looking at the timeline, stumbles & promise to date of the Oxford/AZ trial - https://www.bbc.com/news/health-55308216
& lastly before any 1st step could be taken I wanted to get Wifey on-board. Given the rising #COVID19 cases throughout November & the fact she is a public health-trained RN this wasn’t too hard, but a required step 



To recap, I was:
 reassured about speed of #COVIDvaccine development
reassured about speed of #COVIDvaccine development
 comfortable w/risk:benefit of mRNA vaccines if offered
comfortable w/risk:benefit of mRNA vaccines if offered
 considering 2 trials
considering 2 trials
& decided on Oxford/AZ bc I:
 trusted their scientific process & @ZekeMD
trusted their scientific process & @ZekeMD
 wanted to contribute both locally & globally
wanted to contribute both locally & globally
(21/)
 reassured about speed of #COVIDvaccine development
reassured about speed of #COVIDvaccine development comfortable w/risk:benefit of mRNA vaccines if offered
comfortable w/risk:benefit of mRNA vaccines if offered considering 2 trials
considering 2 trials & decided on Oxford/AZ bc I:
 trusted their scientific process & @ZekeMD
trusted their scientific process & @ZekeMD  wanted to contribute both locally & globally
wanted to contribute both locally & globally(21/)
I was also inspired by several docs who shared their experiences enrolling in a #COVID19 trial including one of my mentors @navsaria - https://madison.com/ct/opinion/column/dr-dipesh-navsaria-i-m-in-a-covid-vaccine-trial-please-think-about-joining-me/article_20037490-f4b0-5d85-95c9-831f71c2f8b0.html
As well as these Black women MDs
@Gradydoctor - https://twitter.com/gradydoctor/status/1324762224192331778?s=21
@DrChrisMD - https://twitter.com/hereandnow/status/1338552334562041862?s=21
As well as these Black women MDs
@Gradydoctor - https://twitter.com/gradydoctor/status/1324762224192331778?s=21
@DrChrisMD - https://twitter.com/hereandnow/status/1338552334562041862?s=21
So on to my Phase 3 clinical trial experience thus far!
Citing another inspiring Black woman MD I’m going to link @drfna’s excellent thread here because there are a lot of similarities that I’m going to try & not dwell on again too much - https://twitter.com/drfna/status/1337814573341151235?s=21
(23/)
Citing another inspiring Black woman MD I’m going to link @drfna’s excellent thread here because there are a lot of similarities that I’m going to try & not dwell on again too much - https://twitter.com/drfna/status/1337814573341151235?s=21
(23/)
I submitted a prescreen questionnaire that asked about my baseline health, occupation/exposure risk, age, gender & race/ethnicity. Based on those answers I was deemed to qualify for participation.
I then scheduled an appointment & was sent a thorough consent packet via email.
I then scheduled an appointment & was sent a thorough consent packet via email.
There I learned that I had a 2 in 3 chance of getting the actual vaccine vs placebo. As well as the frequency with which I’d have to check in-person vs virtually & the planned length of the trial - 2 years.
(25/)
(25/)
I also learned participants get $100 for every in-person visit. Of note, this was not advertised at all.
Bc of a change in my schedule I had to delay my appointment. More info came out about Pfizer/Moderna. I could now wait & likely get a vaccine for sure in months, maybe weeks.
Bc of a change in my schedule I had to delay my appointment. More info came out about Pfizer/Moderna. I could now wait & likely get a vaccine for sure in months, maybe weeks.
However my mind was set. The day came. I showed up to my appointment & answered some of the same questions from the screening. I also asked several of my own:
 were they doing a 1/2 dose 1st shot as part of their protocol given the early results? - No, not yet planned in the US
were they doing a 1/2 dose 1st shot as part of their protocol given the early results? - No, not yet planned in the US
 were they doing a 1/2 dose 1st shot as part of their protocol given the early results? - No, not yet planned in the US
were they doing a 1/2 dose 1st shot as part of their protocol given the early results? - No, not yet planned in the US
 could I get results of my SARS-CoV-2 antibody tests (part of the blood draw they did)? - No, they go to a 3rd party vendor
could I get results of my SARS-CoV-2 antibody tests (part of the blood draw they did)? - No, they go to a 3rd party vendor did I really need another nasal swab PCR (joking, but that was now my 5th one since #COVID19 arrived, not a fan)
did I really need another nasal swab PCR (joking, but that was now my 5th one since #COVID19 arrived, not a fan)(28/)
 any discussions about ending the trial early if efficacy & safety are significant given the ongoing #COVID19 surge to allow those who received placebo to get an actual vaccine? - nothing concrete, they are monitoring closely & participants can leave the trial when they choose
any discussions about ending the trial early if efficacy & safety are significant given the ongoing #COVID19 surge to allow those who received placebo to get an actual vaccine? - nothing concrete, they are monitoring closely & participants can leave the trial when they choose
So after getting clearance to have my pic taken by one of the study coordinators (apparently I was the 1st participant to ask) my arm was prepped & in went the needle!
It was as routine as my annual flu shot (which I got back in September )
)
(30/)
It was as routine as my annual flu shot (which I got back in September
 )
)(30/)
Since then I’ve felt some notable fatigue but otherwise my arm wasn’t even sore (which also is how I feel after the flu shot).
I now answer a questionnaire on an app setup to monitor symptoms & call in any to the clinic. I will then go back in ~30 days for shot #2
I now answer a questionnaire on an app setup to monitor symptoms & call in any to the clinic. I will then go back in ~30 days for shot #2
As vaccine advocates wrestle w/the impact of the medical community’s violation of trust I wanted to heed @CamaraJones words:
“part of being trustworthy is not trying to convince or coax or cajole anybody into taking a vaccine; it is to hear people’s questions & then answer...”
“part of being trustworthy is not trying to convince or coax or cajole anybody into taking a vaccine; it is to hear people’s questions & then answer...”
“...their questions truthfully & clearly, where some of the answers right now may be “We don’t know”
We know a lot more than we did at the beginning, but we have to continue to hold that knowledge w/humility. #COVID19 & 2020 have certainly taught us that https://www.democracynow.org/2020/12/14/camara_phyllis_jones_vaccine_equity
We know a lot more than we did at the beginning, but we have to continue to hold that knowledge w/humility. #COVID19 & 2020 have certainly taught us that https://www.democracynow.org/2020/12/14/camara_phyllis_jones_vaccine_equity
That said, if there is a question or concern I didn’t cover here you are likely to find an answer (or the start of an answer) in this well done, thorough piece: https://elemental.medium.com/every-covid-19-vaccine-question-youll-ever-have-answered-9a0eeb334ded
I truly believe #VaccinesSaveLives.
A #COVIDvaccine trial may not be for you but hopefully everyone, whether it be by masking, socially distancing or getting your own #COVID shot, finds ways to help protect our communities from #COVID19



(End, whew!)
A #COVIDvaccine trial may not be for you but hopefully everyone, whether it be by masking, socially distancing or getting your own #COVID shot, finds ways to help protect our communities from #COVID19



(End, whew!)

 Read on Twitter
Read on Twitter