Couple of notes on the Moderna VRBPAC FDA briefing document. A thread 1/
#VRBPAC #COVID19Vaccine
https://www.fda.gov/media/144434/download
#VRBPAC #COVID19Vaccine
https://www.fda.gov/media/144434/download
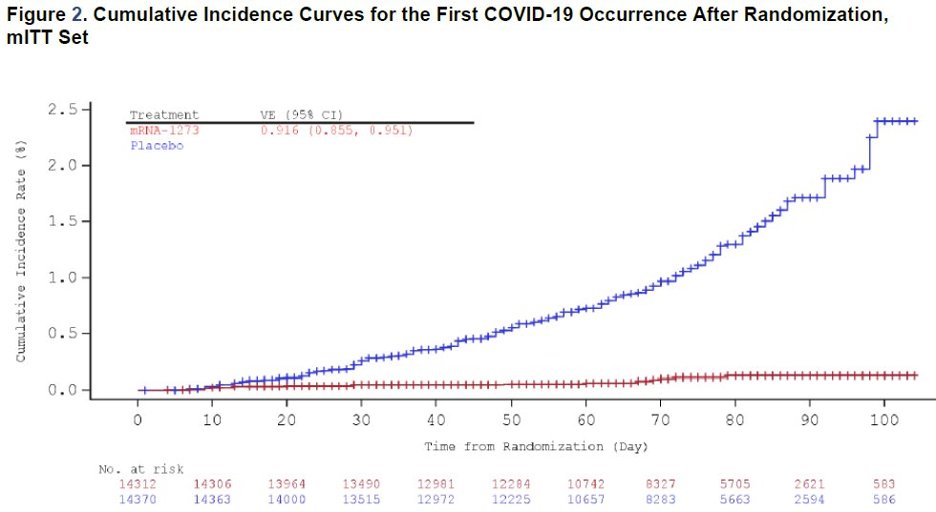
This is just beautiful. Note that the 2nd dose was given at day 28, and curves diverge around day 14. Early protection. 2/
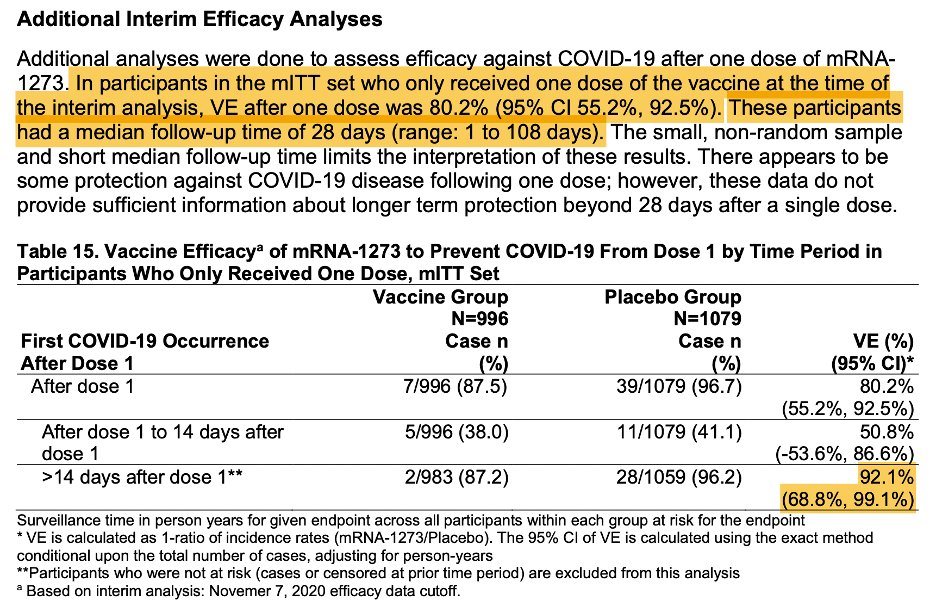
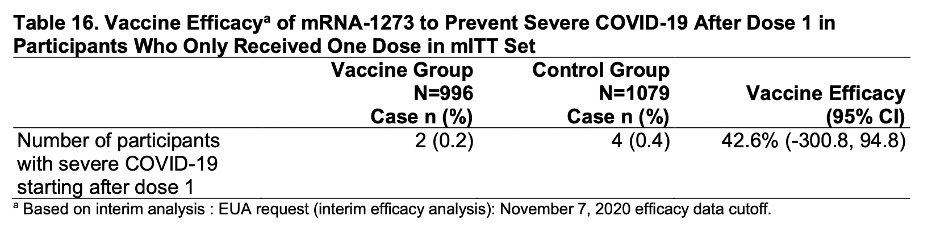
They also give details about the ~2000 subjects who received only one dose. VE 14 days after the 1st dose was 92% (wide CI though) and 80.2% overall. 3/
Also note the short follow-up- median of 28 days, which makes sense bc that’s when the 2nd dose they refused was scheduled. I think we can conclude that protection starts early, but don’t know its durability. 2 doses should be the regimen. 5/
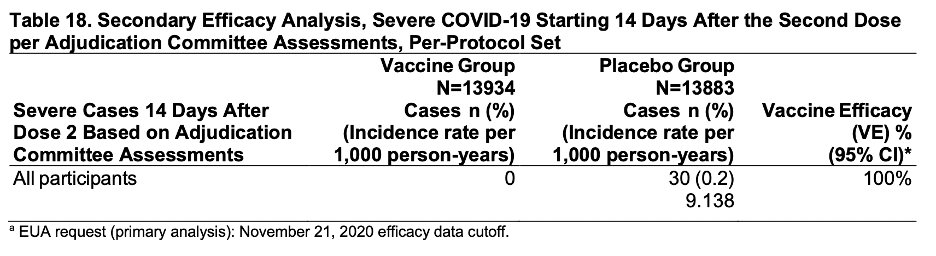
For the secondary endpoint of severe cases 14 days after the 2nd dose, there were no cases in the vaccine group vs. 30 in placebo (9 hospitalized). That’s 100% efficacy, my friends. 6/
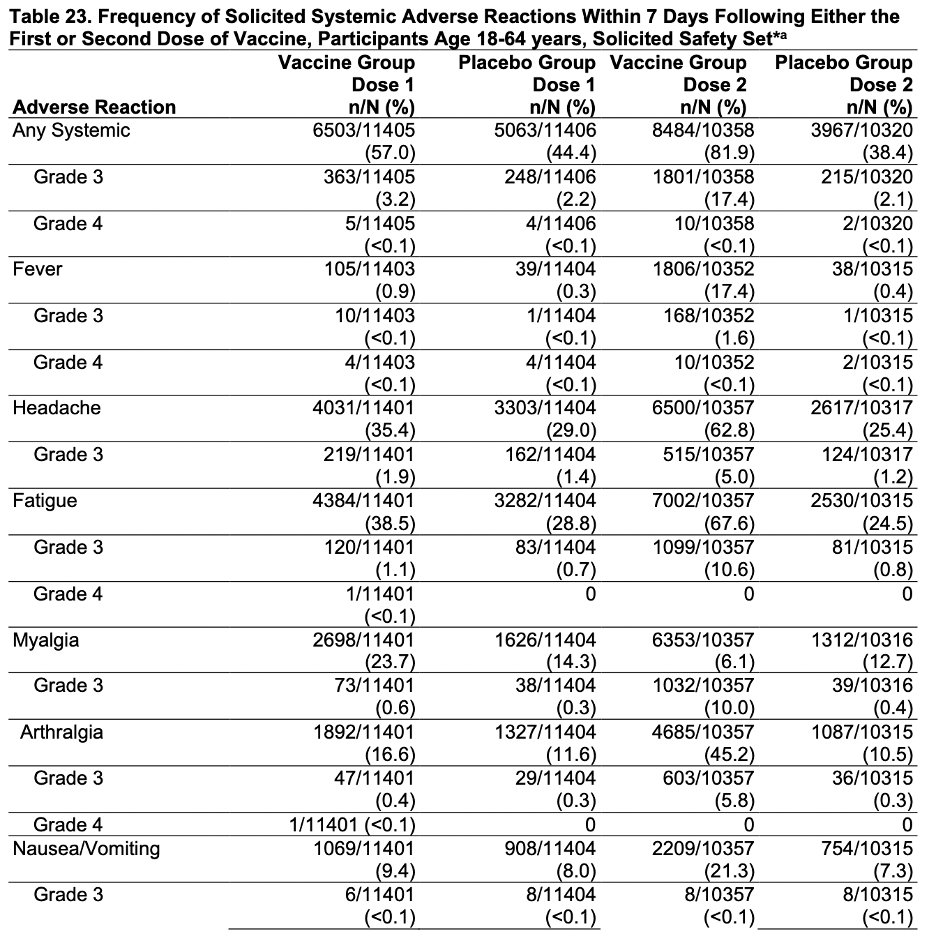
Safety is reported in a myriad of ways. Fatigue, fever (17% after dose 2), headache, and myalgias were common. We need to warn patient about this and not downplay them. All were less common in >=65 YO, who either had less acute immune responses or complained less. 7/
Pregnancy occurred in 6 vaccinated patients and 7 placebo patients. No known outcomes yet. 9/
Overall, this looks like another home run – high efficacy, common but moderate adverse effects. I would take it and recommend it. We are very fortunate that these 2 (so far) vaccines have worked out. Now I can finally pin this to my lapel, and @jpogue1 can shave! 10/

 Read on Twitter
Read on Twitter