Thread about $XENE Xenon Pharmceuticals‘ main asset XEN1101: XEN1101 is a – presumably – much improved version of an older approved drug: ezogabine (= retigabine; trade names EU/USA Trobalt/Potiga).
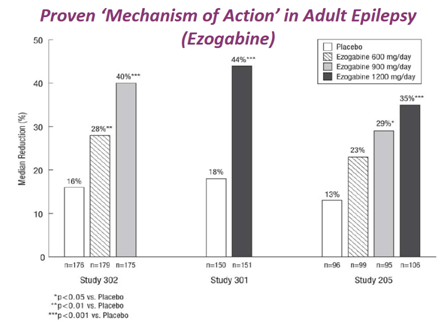
This drug is a KV7 potassium channel modulator and was approved for adult focal onset seizures. Median seizure reduction as per chart (in line with most current AEDs anti-epileptic drugs)
Due to issues with pigmentation e.g. in the eyes, and due to suspected risk of vision loss (which did not prove to be the case later!), the drug got a black box. The REMS requirement was dropped in 2015 because no tox issues were found, but the damage was done, and GSK/Valeant
pulled the drug from the market in July 2017 for commercial reasons. XEN1101 has the same mechanism of action as ezogabine and was developed by the same people at Vaelant (Christopher Crean). It was licensed in 2017 by Xenon at favorable conditions (see amended conditions below)
XEN1101 is potentially a considerably improved drug to ezogabine: 1. It will most likely completely avoid the pigmentation/discoloration issues seen with ezogabine 2. Considerably more potent and more selective drug in several models 3. No need for titration 4. Convenient
once daily pill evening dosing (ezogabine was 3/day). These characteristics would make it attractive in the epilepsy market, assuming good safety and ~same efficacy as other AEDs (~35%-45% range, and outlier is cenobamate with ~50-55% but this one also has titration requirement).
Phase 1b studies were only done in healthy volunteers, so we have not seen any efficacy data in human yet for XEN1101! Though there are hints that effectiveness could come in better than ezogabine. Eg. TMS-tests (transcranial magnetic stimulation, which can be used to measure the
consequences of AEDs on cortical excitability) showed ~ 2x the effect on resting motor treshold (RMT) of the 20mg XEN1101 compared to the 400mg ezogabine dose (caveat: cross trial TMS comparison). See https://www.xenon-pharma.com/wp-content/uploads/2019/12/Beatch_Poster_72x42-FINALi.pdf
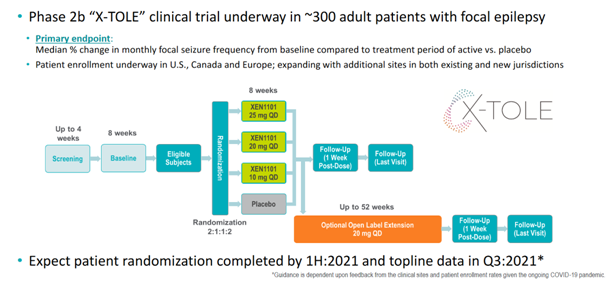
A n=300 P2b study is underway. X-Tole was planned for readout in 2020, but topline guidance got delayed to Q3 2021 due to C19. See chart.
There are TWO IMPORTANT HINTS that this study could indeed succeed (efficacy/safety): 1. The dropout rate is lower than the company expected, and this despite Covid (study was obviously planned before Covid) 2. More than 90% of trial participants moved over into the
52 weeks OLE study – hardly imaginable if the drug does nothing and/or has a lot of adverse events. No guarantees for success, but promising!
Additional indications for XEN1101 are being evaluated, and I assume that also pediatric indications are planned once it gets approved in adolescents.
Here the link to several posters on XEN1101, partly showing preclinical direct comparisons to ezogabine: https://www.xenon-pharma.com/medical-affairs/publications/#XEN1101
Here the link to several posters on XEN1101, partly showing preclinical direct comparisons to ezogabine: https://www.xenon-pharma.com/medical-affairs/publications/#XEN1101
There are several other programs in the pipeline not considered here (XEN007 with promising signal in CAE last week, XEN496 and a few outlicensed drugs) that would mitigate a stock-crash in case of negative XEN1101 results somewhat.
The company has cash runway into 2023 and a low EV of currently < $250m (the Canada discount I guess). All in all I think it can be a good investment for people with patience. 2021 will be more busy than this and last year for sure.
BUT remember: things sometimes go wrong and don't work out as planned, especially in neurology! Also I am a layman and could be very wrong. So good luck and do your own DD! $XENE

 Read on Twitter
Read on Twitter