$CAPR 1/n here’s why I think Accelerated approval coming for dmd indication using cap-1002. See fda guidance: 1.0 point difference will be sufficient to support bla approval.
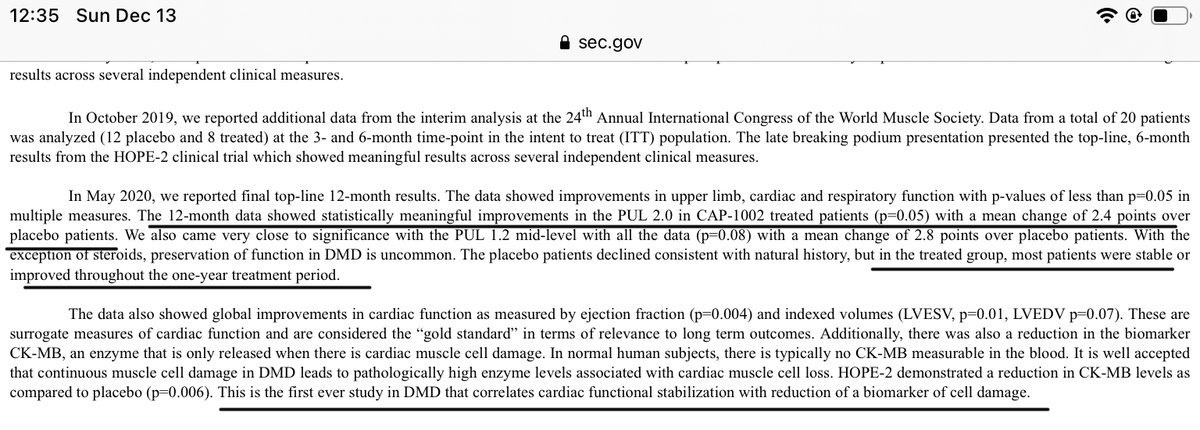
$CAPR 2/n HOPE II trial reported 2.4 point improvement over placebo patients (recall fda asked for 1.0 improvement). In addition, CAPR reported some additional good guy from the trial
$CAPR 3/n accelerated approval (AA) hasn’t happened yet but meanwhile, company has continued to say they’re in discussion with fda on path fwd to bla approval for dmd.
Also, they’ve hired loads of people (check linkedin) that relate to mfg capabilities, quality control etc
Also, they’ve hired loads of people (check linkedin) that relate to mfg capabilities, quality control etc
$CAPR 4/n co has also stated in sec filing that they have initiated tech transfer process with Lonza group for commercial scale mfg process for cap-1002. Why would they do that unless they expect Lonza documentation will be part of bla submission?
$CAPR recall that AA if granted will come with a priority review voucher that the co can retail at around $300M. Current mkt cap is ~$80M with around $35M in cash and no debt. Capital structure is clean.
Last qtr co raised under $3M in atm so they clearly don’t seem to in rush
Last qtr co raised under $3M in atm so they clearly don’t seem to in rush
$CAPR 6/n not to mention all the other work they’re doing in COVID 19 therapeutic (phase 2 enrolling in 2 centers), exosome platform, Pre clinical data in COVID 19 vaccines etc.
Dmd is why I’ve been following them and to be the largest near term value driver still imo
Dmd is why I’ve been following them and to be the largest near term value driver still imo
$CAPR chart sitting at support and small float with ton of upside

 Read on Twitter
Read on Twitter