1/17
Why do patients with advanced heart failure often develop the Cheyne-Stokes breathing pattern?
To understand this phenomenon we'll have to explore circulatory flow time and the concept of loop gain.
And no this tracing isn't Torsades de Pointes
#tweetorial #medtwitter
Why do patients with advanced heart failure often develop the Cheyne-Stokes breathing pattern?
To understand this phenomenon we'll have to explore circulatory flow time and the concept of loop gain.
And no this tracing isn't Torsades de Pointes

#tweetorial #medtwitter
2/
What exactly is Cheyne-Stokes respiration (CSR)?
 This respiratory pattern is characterized by tachypnea and hyperpnea (aka deep breathing) alternating with periods of apnea.
This respiratory pattern is characterized by tachypnea and hyperpnea (aka deep breathing) alternating with periods of apnea.
The pattern then repeats cyclically.
https://pubmed.ncbi.nlm.nih.gov/10228116/
What exactly is Cheyne-Stokes respiration (CSR)?
 This respiratory pattern is characterized by tachypnea and hyperpnea (aka deep breathing) alternating with periods of apnea.
This respiratory pattern is characterized by tachypnea and hyperpnea (aka deep breathing) alternating with periods of apnea. The pattern then repeats cyclically.
https://pubmed.ncbi.nlm.nih.gov/10228116/
3/
Let's start w/ some history.
CSR was actually first described by the Hippocratic authors (400 BCE).
Observing the illness of a man named Philiscus, it was noted that "his respiration [was] like that of a person recollecting himself, rare and large".
http://classics.mit.edu/Hippocrates/epidemics.1.i.html
Let's start w/ some history.
CSR was actually first described by the Hippocratic authors (400 BCE).
Observing the illness of a man named Philiscus, it was noted that "his respiration [was] like that of a person recollecting himself, rare and large".
http://classics.mit.edu/Hippocrates/epidemics.1.i.html
4/
John Cheyne and William Stokes, for whom the breathing pattern is named, were 19th century Scottish and Irish physicians, respectively.
They each independently described this pattern in patients dying of heart failure.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5661788/
John Cheyne and William Stokes, for whom the breathing pattern is named, were 19th century Scottish and Irish physicians, respectively.
They each independently described this pattern in patients dying of heart failure.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5661788/
5/
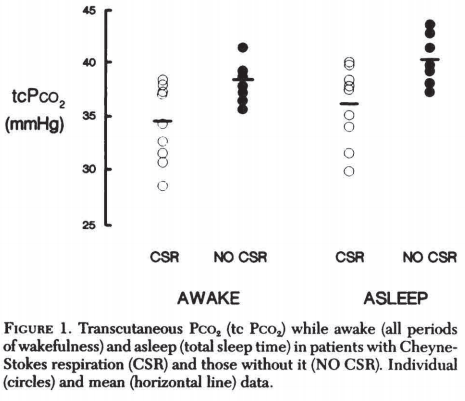
So how does heart failure lead to CSR?
The inciting event seems to be chronic hypocapnia, which is common in advanced CHF (due to chronic congestion, among other reasons).
Note that those w/ CSR had consistently pCO₂ levels in this 1993 study.
pCO₂ levels in this 1993 study.
https://pubmed.ncbi.nlm.nih.gov/8404170/
So how does heart failure lead to CSR?
The inciting event seems to be chronic hypocapnia, which is common in advanced CHF (due to chronic congestion, among other reasons).
Note that those w/ CSR had consistently
 pCO₂ levels in this 1993 study.
pCO₂ levels in this 1993 study. https://pubmed.ncbi.nlm.nih.gov/8404170/
6/
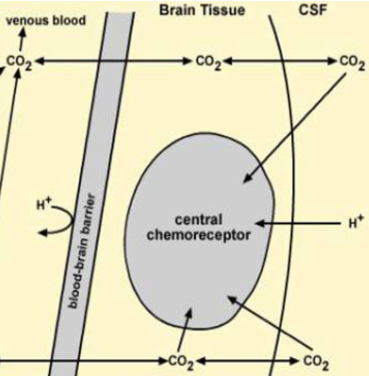
Next we need to understand how CO₂ levels affect respiration.
CO₂ levels affect respiration.
While there are many inputs to the medullary respiratory control center, hypocapnia leads to:
 CSF pH
CSF pH
 central chemoreceptor stimulation and
central chemoreceptor stimulation and
 respiratory rate (or even apnea).
respiratory rate (or even apnea).
https://www.sciencedirect.com/science/article/pii/B9780080552323603150
Next we need to understand how
 CO₂ levels affect respiration.
CO₂ levels affect respiration.While there are many inputs to the medullary respiratory control center, hypocapnia leads to:
 CSF pH
CSF pH central chemoreceptor stimulation and
central chemoreceptor stimulation and respiratory rate (or even apnea).
respiratory rate (or even apnea).https://www.sciencedirect.com/science/article/pii/B9780080552323603150
7/
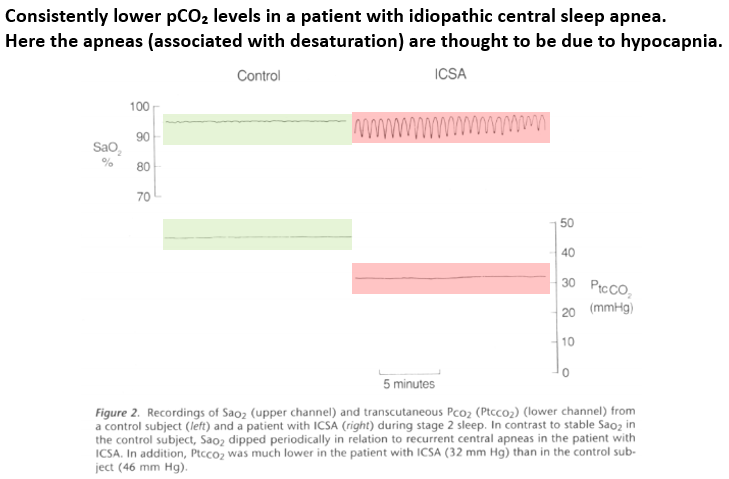
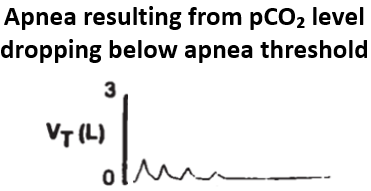
Let's zoom in on apneas w/ hypocapnia.
The respiratory control center has a serum CO₂ level below which apnea results, aka the apnea threshold.
Those w/ chronic hypocapnia often have CO₂ levels just above their threshold, predisposing to apneas.
https://pubmed.ncbi.nlm.nih.gov/8520761/
Let's zoom in on apneas w/ hypocapnia.
The respiratory control center has a serum CO₂ level below which apnea results, aka the apnea threshold.
Those w/ chronic hypocapnia often have CO₂ levels just above their threshold, predisposing to apneas.
https://pubmed.ncbi.nlm.nih.gov/8520761/
8/
So the apnea component of CSR makes sense:
 If already close to the apnea threshold, a patient's serum CO₂ level wouldn't have to drop much to go below this threshold.
If already close to the apnea threshold, a patient's serum CO₂ level wouldn't have to drop much to go below this threshold.
But what about the tachypnea/hyperpnea phase of CSR?
So the apnea component of CSR makes sense:
 If already close to the apnea threshold, a patient's serum CO₂ level wouldn't have to drop much to go below this threshold.
If already close to the apnea threshold, a patient's serum CO₂ level wouldn't have to drop much to go below this threshold.But what about the tachypnea/hyperpnea phase of CSR?
9/
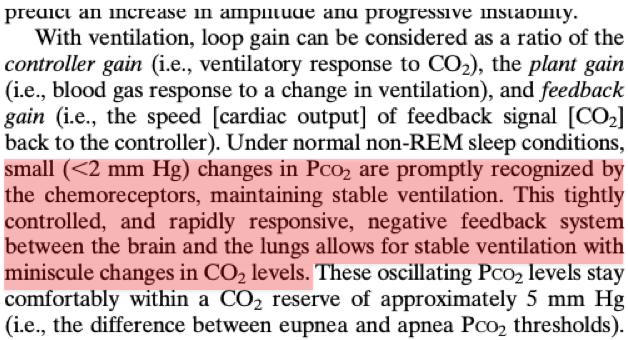
As we learned in tweet #6, changes in pCO₂ lead to adjustments in respiration to compensate.
Under normal conditions, these pCO₂/respiratory changes are small and rapid.
In other words, the pCO₂-ventilatory relationship is stable.
https://pubmed.ncbi.nlm.nih.gov/20053968/
As we learned in tweet #6, changes in pCO₂ lead to adjustments in respiration to compensate.
Under normal conditions, these pCO₂/respiratory changes are small and rapid.
In other words, the pCO₂-ventilatory relationship is stable.
https://pubmed.ncbi.nlm.nih.gov/20053968/
10/
But things are different in patients with advanced CHF.
 Decreased cardiac output causes the blood of such patients to circulate more slowly compared to those w/ normal cardiac function.
Decreased cardiac output causes the blood of such patients to circulate more slowly compared to those w/ normal cardiac function.
In this 1933 study the circulation time was 26 vs 15 seconds.
https://bit.ly/36UOU9q
But things are different in patients with advanced CHF.
 Decreased cardiac output causes the blood of such patients to circulate more slowly compared to those w/ normal cardiac function.
Decreased cardiac output causes the blood of such patients to circulate more slowly compared to those w/ normal cardiac function.In this 1933 study the circulation time was 26 vs 15 seconds.
https://bit.ly/36UOU9q
11/
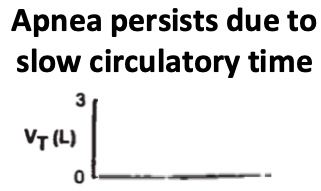
Normally, during apnea the respiratory control center senses rising pCO₂ and ends the apnea, resuming normal breathing.
But in advanced CHF, increased circulatory time renders this pCO₂-ventilatory relationship unstable.
Normally, during apnea the respiratory control center senses rising pCO₂ and ends the apnea, resuming normal breathing.
But in advanced CHF, increased circulatory time renders this pCO₂-ventilatory relationship unstable.
12/
During an apnea in a patient w/ advanced CHF, pCO₂ increases but the respiratory control center doesn't sense this right away (due to circulatory times).
circulatory times).
A pCO₂-breathing disconnect results, as the brain can't quickly respond to elevated pCO₂. https://pubmed.ncbi.nlm.nih.gov/26972039/
During an apnea in a patient w/ advanced CHF, pCO₂ increases but the respiratory control center doesn't sense this right away (due to
 circulatory times).
circulatory times).A pCO₂-breathing disconnect results, as the brain can't quickly respond to elevated pCO₂. https://pubmed.ncbi.nlm.nih.gov/26972039/
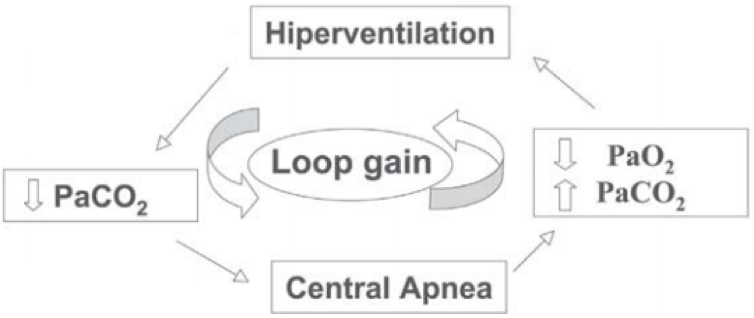
13/
Overcompensation ensues, as the brain eventually responds to hypercapnia w/ tachypnea + hyperpnea.
This drives pCO₂ down and the cycle repeats.
The technical term for this phenomenon is loop gain (a concept borrowed from electrical circuitry).
https://pubmed.ncbi.nlm.nih.gov/16138241/
Overcompensation ensues, as the brain eventually responds to hypercapnia w/ tachypnea + hyperpnea.
This drives pCO₂ down and the cycle repeats.
The technical term for this phenomenon is loop gain (a concept borrowed from electrical circuitry).
https://pubmed.ncbi.nlm.nih.gov/16138241/
14/
Let’s use a hypothetical example of CSR to put this all together.
A patient w/ advanced CHF has a baseline pCO₂ level of 32 mm Hg and an apnea threshold of 30 mm Hg.
The pCO₂ drifts down below 30 mm Hg and an apnea results.
Let’s use a hypothetical example of CSR to put this all together.
A patient w/ advanced CHF has a baseline pCO₂ level of 32 mm Hg and an apnea threshold of 30 mm Hg.
The pCO₂ drifts down below 30 mm Hg and an apnea results.
15/
pCO₂ rises during the subsequent apnea, but this is not immediately sensed by the respiratory control center because of slow circulatory time.
As a result, the apnea phase persists longer than it normally would and the pCO₂ continues to rise.
pCO₂ rises during the subsequent apnea, but this is not immediately sensed by the respiratory control center because of slow circulatory time.
As a result, the apnea phase persists longer than it normally would and the pCO₂ continues to rise.
16/
Eventually the respiratory control center senses hypercapnia and responds by inducing tachypnea and hyperpnea.
Increased respiratory rate and tidal volume drive pCO₂ back down to below the apnea threshold and the cycle continues.
 This is Cheyne-Stokes respiration.
This is Cheyne-Stokes respiration.
Eventually the respiratory control center senses hypercapnia and responds by inducing tachypnea and hyperpnea.
Increased respiratory rate and tidal volume drive pCO₂ back down to below the apnea threshold and the cycle continues.
 This is Cheyne-Stokes respiration.
This is Cheyne-Stokes respiration.
17/
 Cheyne-Stokes respiration is triggered by chronic hypocapnia and low cardiac output
Cheyne-Stokes respiration is triggered by chronic hypocapnia and low cardiac output
 Hypocapnia
Hypocapnia  apneic episodes, while low cardiac output
apneic episodes, while low cardiac output  slow circulatory flow and delayed responses to changes in pCO₂
slow circulatory flow and delayed responses to changes in pCO₂
 This results in a cycle of apnea and deep/rapid breathing
This results in a cycle of apnea and deep/rapid breathing
 Cheyne-Stokes respiration is triggered by chronic hypocapnia and low cardiac output
Cheyne-Stokes respiration is triggered by chronic hypocapnia and low cardiac output Hypocapnia
Hypocapnia  apneic episodes, while low cardiac output
apneic episodes, while low cardiac output  slow circulatory flow and delayed responses to changes in pCO₂
slow circulatory flow and delayed responses to changes in pCO₂ This results in a cycle of apnea and deep/rapid breathing
This results in a cycle of apnea and deep/rapid breathing
Thanks to @Shariqqwani for catching an oversight in tweet #6. Hypocapnia will raise CSF pH, not decrease.

 Read on Twitter
Read on Twitter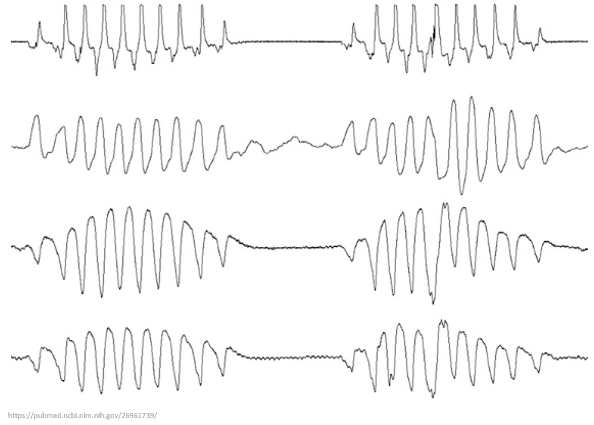

![3/Let's start w/ some history.CSR was actually first described by the Hippocratic authors (400 BCE).Observing the illness of a man named Philiscus, it was noted that "his respiration [was] like that of a person recollecting himself, rare and large". http://classics.mit.edu/Hippocrates/epidemics.1.i.html 3/Let's start w/ some history.CSR was actually first described by the Hippocratic authors (400 BCE).Observing the illness of a man named Philiscus, it was noted that "his respiration [was] like that of a person recollecting himself, rare and large". http://classics.mit.edu/Hippocrates/epidemics.1.i.html](https://pbs.twimg.com/media/EpITn5oW8AYl-6v.png)