My 1st Thread!
Some of my comments/questions re: Pfizer COVID Vaccine.
***For the record, I am a pro-vaccines***
Sources:
> FDA Briefing Document
https://www.fda.gov/media/144245/download
> NEJM Article https://www.nejm.org/doi/pdf/10.1056/NEJMoa2034577
> FDA Vaccine Advisory Committee (VRBPAC) on 12/10/2020
Some of my comments/questions re: Pfizer COVID Vaccine.
***For the record, I am a pro-vaccines***
Sources:
> FDA Briefing Document
https://www.fda.gov/media/144245/download
> NEJM Article https://www.nejm.org/doi/pdf/10.1056/NEJMoa2034577
> FDA Vaccine Advisory Committee (VRBPAC) on 12/10/2020
General Design: Vax (inj on Day 0 & Day 21) vs Placebo.
Participants #: ~20k vs ~20k= ~40,000
Outcome (comparison): COVID cases at least 7 days after 2nd injection (after day #28 + ~1 more month)
*KEY POINT* COVID = Disease **w/ symptoms** (not just a positive virus test)
Participants #: ~20k vs ~20k= ~40,000
Outcome (comparison): COVID cases at least 7 days after 2nd injection (after day #28 + ~1 more month)
*KEY POINT* COVID = Disease **w/ symptoms** (not just a positive virus test)
"Previous SARS-CoV-2 Infxn" (briefing doc)
Visit #1 (D0), ~3% positive (+ hx or asymptomatic) 526/18163 [2.9%] vs 567/18287 [3.1%])(p27)
"Outcome" day (~D28): pts w/o evid of infxn prior to 7d after dose #2: ~86% (p18); therefore, this means ~14% asymptomatic positive, correct?
Visit #1 (D0), ~3% positive (+ hx or asymptomatic) 526/18163 [2.9%] vs 567/18287 [3.1%])(p27)
"Outcome" day (~D28): pts w/o evid of infxn prior to 7d after dose #2: ~86% (p18); therefore, this means ~14% asymptomatic positive, correct?
Serious questions (honestly not sure):
Am I interpreting correctly that the asymptomatic positivity rate went from ~3% to ~14% (evenly matched in both arms) in ~28 days?
If accurate, then would it be fair to suggest that the *vaccine did not prevent asymptomatic carriers*?
Am I interpreting correctly that the asymptomatic positivity rate went from ~3% to ~14% (evenly matched in both arms) in ~28 days?
If accurate, then would it be fair to suggest that the *vaccine did not prevent asymptomatic carriers*?
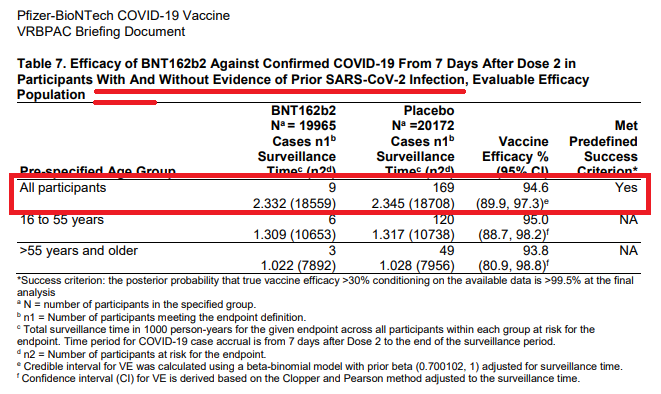
Primary Outcome [+ COVID (symptomatic disease)]
Pts w/o the asymptomatic carriers on ~D28 (7d after 2nd dose): 8 vs 162 (vax vs placebo respectively)(p24).
"Vaccine Efficacy" (VE) = 154/162 = 95% didn't get COVID in vax arm compared to placebo arm.
Pts w/o the asymptomatic carriers on ~D28 (7d after 2nd dose): 8 vs 162 (vax vs placebo respectively)(p24).
"Vaccine Efficacy" (VE) = 154/162 = 95% didn't get COVID in vax arm compared to placebo arm.
Question:
Is "Vaccine Efficacy" another way to say "Relative Risk Reduction"?
Vax: 8/17411 = 0.046%
Placebo: 162/17511 = 0.925%
"Absolute" Risk Reduction = 0.88%
"Relative" Risk Reduction = 95% (0.88/0.925)
Is it fair to say that getting the vax reduces risk of COVID by ~1%?
Is "Vaccine Efficacy" another way to say "Relative Risk Reduction"?
Vax: 8/17411 = 0.046%
Placebo: 162/17511 = 0.925%
"Absolute" Risk Reduction = 0.88%
"Relative" Risk Reduction = 95% (0.88/0.925)
Is it fair to say that getting the vax reduces risk of COVID by ~1%?
What if you add in those pts with a known or unknown history of the virus (which likely would reflect the population to get vaccinated)?
Basically the same results (p25)
Vax: 9/18559 = 0.048%
Placebo: 169/18708 = 0.903%
"Absolute" RR = 0.86%
"Relative" RR = 94.7% (0.855/0.903)
Basically the same results (p25)
Vax: 9/18559 = 0.048%
Placebo: 169/18708 = 0.903%
"Absolute" RR = 0.86%
"Relative" RR = 94.7% (0.855/0.903)
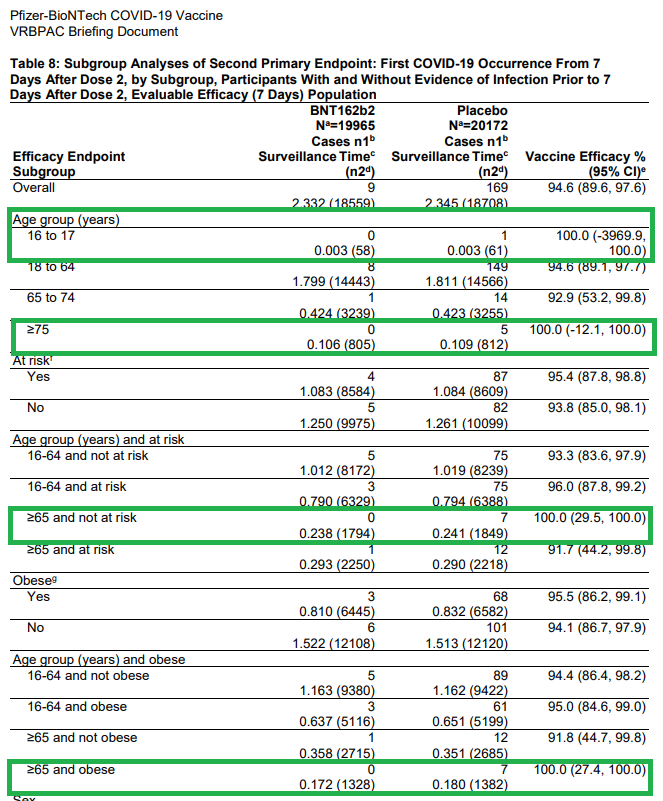
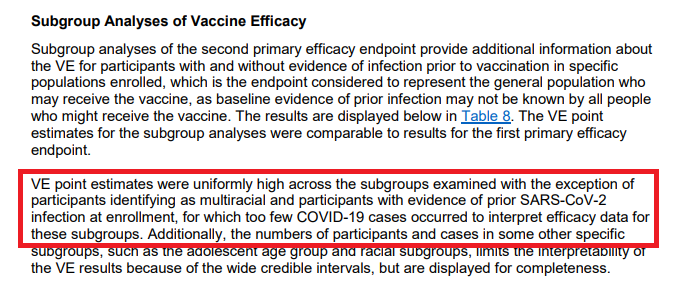
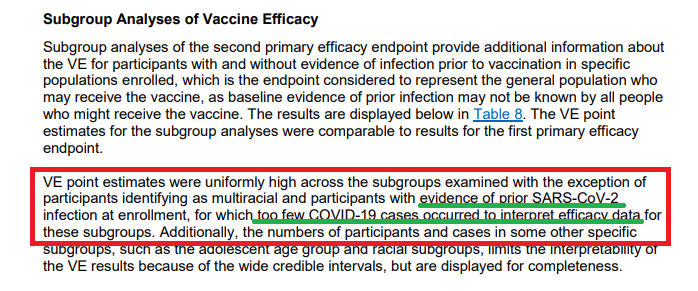
What about subgroups? This is where I get a bit frustrated (double standard when the # isn't favorable?).
Per the statement on p25, VE is high across all subgroups with the exception for multiracial or previous SARS infection at enrollment.
Wow! What a statement!
Per the statement on p25, VE is high across all subgroups with the exception for multiracial or previous SARS infection at enrollment.
Wow! What a statement!
So, let me get this right.
If the VE is a good number (100%), regardless of the low denominator, then we'll include them to say how effective the vax is in these patients.
However, if the number is bad (10.4% or -7.1%), we'll blame it on the low numbers.
If the VE is a good number (100%), regardless of the low denominator, then we'll include them to say how effective the vax is in these patients.
However, if the number is bad (10.4% or -7.1%), we'll blame it on the low numbers.
So, there was only 1 COVID+ case in the placebo arm for multiracial and previous SARS+ infxn when enrolled, so can't rely on the numbers (note: vax=1; VE=0%).
However, it's OK to include the 1 positive COVID case in the American Indian/Alaska native or Hawaiian (vax=0; VE=100%)
However, it's OK to include the 1 positive COVID case in the American Indian/Alaska native or Hawaiian (vax=0; VE=100%)
Same concept, is it really fair to suggest "high efficacy" for other subgroups when the denominator (COVID+ in placebo arm) is < 10?
16-17yo: n=1
75yo+: n=5
65+ & no risk factors: n=7
65+ & obese: n=7
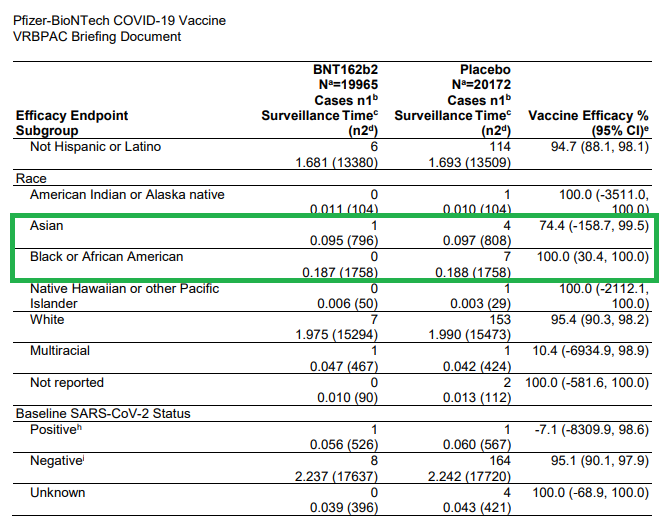
Asian: n=4
Black/AA: n=7
Interesting VE=100% (except Asian n=1; VE=74%)
16-17yo: n=1
75yo+: n=5
65+ & no risk factors: n=7
65+ & obese: n=7
Asian: n=4
Black/AA: n=7
Interesting VE=100% (except Asian n=1; VE=74%)
Interesting that Pfizer submitted EUA to include:
Pts who had prior SARS infection (n=1 comparator)
Despite the statement "...too few COVID cases occurred to interpret efficacy data for these subgroups"
Relied on the 85% non-infected pts to boost up these stats.
Pts who had prior SARS infection (n=1 comparator)
Despite the statement "...too few COVID cases occurred to interpret efficacy data for these subgroups"
Relied on the 85% non-infected pts to boost up these stats.
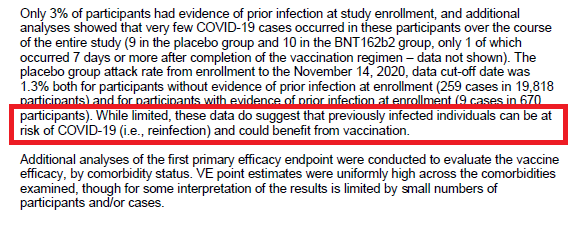
And is it fair to include the statement "While limited, these data do suggest that previously infected individuals can be at risk of COVID and could benefit from vax". (p28)
Where are the data to show the vaccine benefit (if n=1 in both arms) and based on your other statement?
Where are the data to show the vaccine benefit (if n=1 in both arms) and based on your other statement?
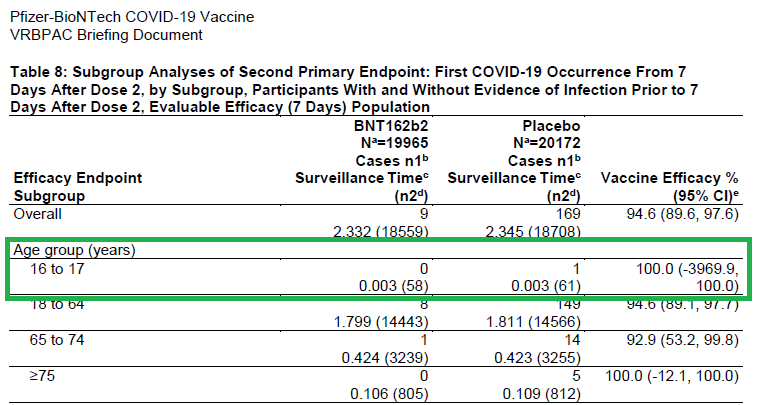
Interesting that Pfizer submitted EUA to include:
16-17yo (despite n=1 comparator - look at that confidence interval!).
Lots of discussion from the Vaccine Advisory Committee about this age group - likely the rationale for most (all?) of those who voted "No" or "Abstain".
16-17yo (despite n=1 comparator - look at that confidence interval!).
Lots of discussion from the Vaccine Advisory Committee about this age group - likely the rationale for most (all?) of those who voted "No" or "Abstain".
Related to younger patients, a statement on p15 concerned me (regarding 100 pts ages 12-15):
"...the available data were insufficient to support a FAVORABLE benefit-risk determination at this time."
Is there something they know but haven't shared with us?
"...the available data were insufficient to support a FAVORABLE benefit-risk determination at this time."
Is there something they know but haven't shared with us?
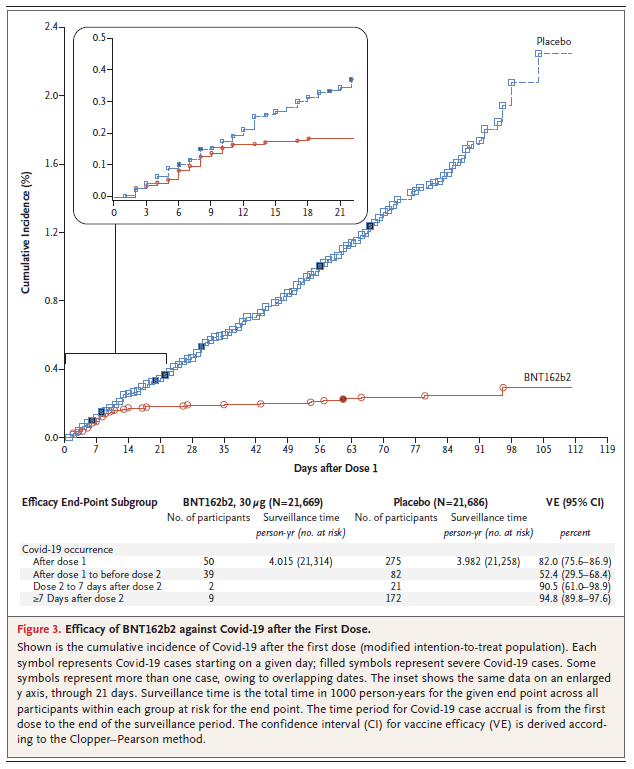
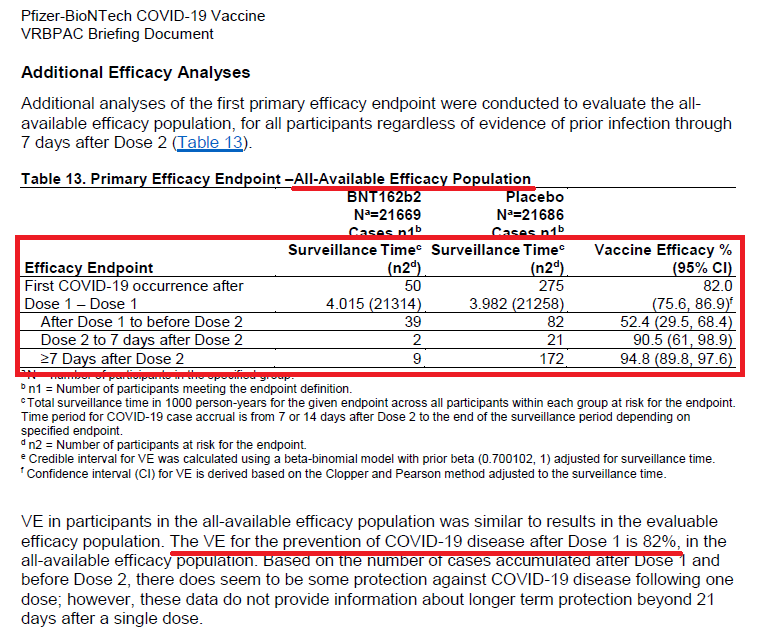
This was an encouraging graph, suggesting that vaccine efficacy (immunity) may be beneficial starting ~D14 (only 1 dose).
Of note, this graph does incorporate the 2nd dose being given ~D21.
The 2nd dose is necessary to sustain immunity; however, ?flexibility if given > 21 days?
Of note, this graph does incorporate the 2nd dose being given ~D21.
The 2nd dose is necessary to sustain immunity; however, ?flexibility if given > 21 days?
Of note, they do state VE after Dose 1 is 82% in the all-available efficacy population with the following VE breakdown:
D0-21: 52.4%
D21-D28 (2-7d after 2nd dose): 90.5%
D28+ (7d+ after 2nd dose): 94.8%
D0-21: 52.4%
D21-D28 (2-7d after 2nd dose): 90.5%
D28+ (7d+ after 2nd dose): 94.8%
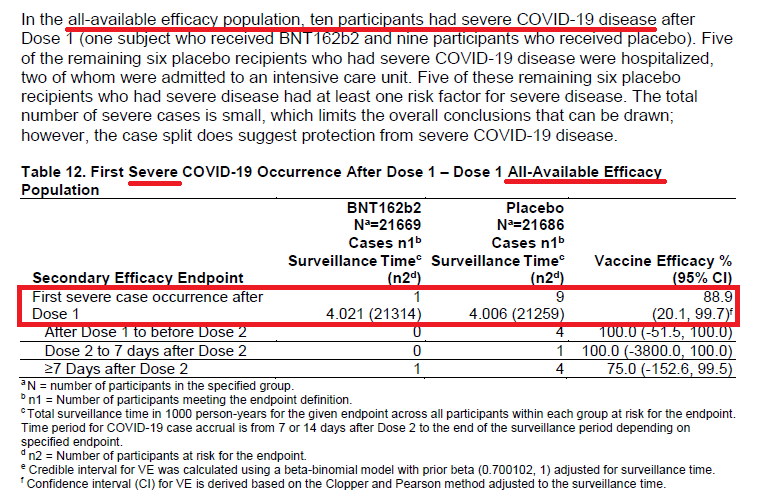
Secondary Endpoint: "Severe" COVID.
"Severe" per FDA definition (p14).
Interesting that out of almost 44,000 patients (in the All-Available population), that only 10 patients were "severe" during this one-month period. (p31)
"Severe" per FDA definition (p14).
Interesting that out of almost 44,000 patients (in the All-Available population), that only 10 patients were "severe" during this one-month period. (p31)
Dr. Sheldon Toubman (of the Vaccine Advisory Committee) commented that although it's great to prevent pts from getting the mild/mod symptoms (cough, etc.), it's the SEVERE COVID is really the most important.
Yet, with only 10 pts having severe; can we really determine efficacy?
Yet, with only 10 pts having severe; can we really determine efficacy?
Similar to the subgroup analyses...
Is it appropriate to determine vaccine effectiveness when using a denominator of 3 and 9, depending on which population you are analyzing?
Is it appropriate to determine vaccine effectiveness when using a denominator of 3 and 9, depending on which population you are analyzing?
Similar to the above...
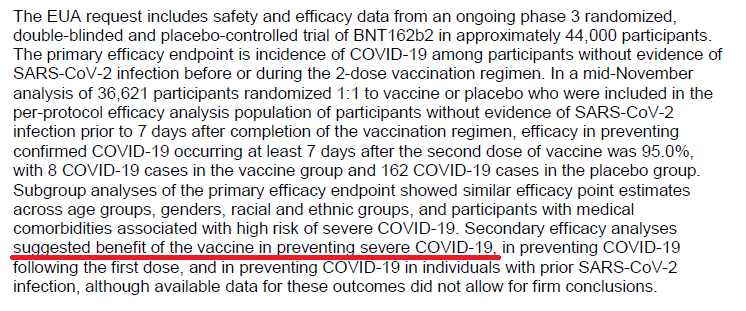
Is it really fair to make the statement "...Secondary efficacy analyses suggested benefit of the vax in preventing severe COVID..." (quoted from the Executive Summary) when there were only 10 pts (out of 44,000) & denominator of 3 (evaluable population)?
Is it really fair to make the statement "...Secondary efficacy analyses suggested benefit of the vax in preventing severe COVID..." (quoted from the Executive Summary) when there were only 10 pts (out of 44,000) & denominator of 3 (evaluable population)?
Safety:
Some very frequent (yet non-severe) reactogenicity adverse events (AE); however, I felt Pfizer has been very transparent and these are being well communicated.
Personally, I feel patients should be educated on these - "prepare, not scare".
Some very frequent (yet non-severe) reactogenicity adverse events (AE); however, I felt Pfizer has been very transparent and these are being well communicated.
Personally, I feel patients should be educated on these - "prepare, not scare".
Local (injection-site-related) adverse events:
Pain @ injection site:
55yo & younger: ~80%
> 55 yo: ~70%
2nd Injection? Slightly better than 1st one.
Redness/swelling? Not too bad.
Pain @ injection site:
55yo & younger: ~80%
> 55 yo: ~70%
2nd Injection? Slightly better than 1st one.
Redness/swelling? Not too bad.
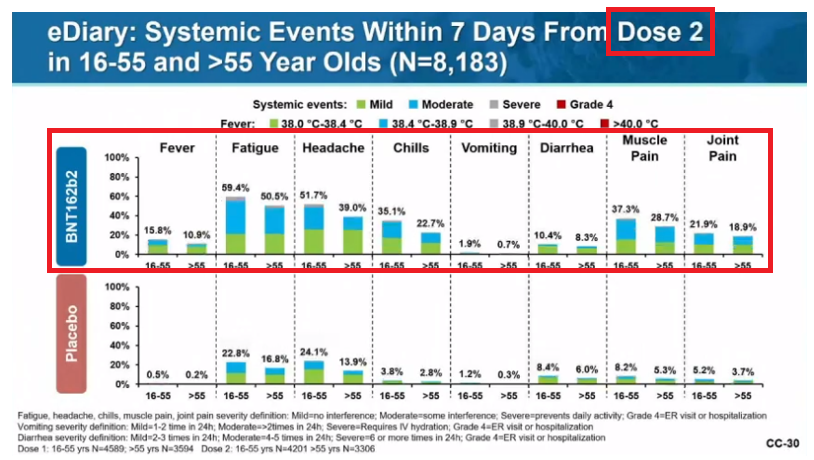
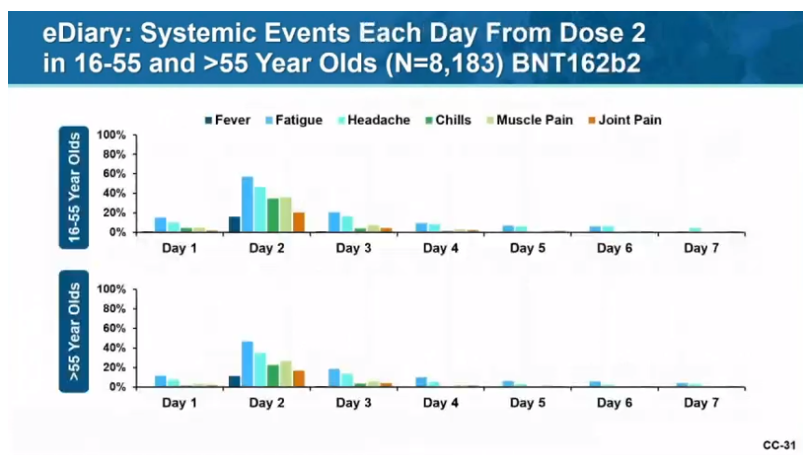
Systemic adverse events:
Fatigue: 35-60% (2nd inj worse than 1st; older tolerate better than younger)
Headache: 25-52% (2nd inj worse than 1st; older tolerate better)
Muscle pain: 29-37% w/ 2nd inj (older tolerate better)
Chills: 23-35% w/ 2nd inj (older tolerate better).
Fatigue: 35-60% (2nd inj worse than 1st; older tolerate better than younger)
Headache: 25-52% (2nd inj worse than 1st; older tolerate better)
Muscle pain: 29-37% w/ 2nd inj (older tolerate better)
Chills: 23-35% w/ 2nd inj (older tolerate better).
When should these be expected and for how long?
Great graph here.
Onset? 1 to 2 days (median)
Duration? 1 day (median)
Great graph here.
Onset? 1 to 2 days (median)
Duration? 1 day (median)
Given the relatively high frequency to have a NON-SERIOUS adverse event, seems reasonable to try and schedule some time off for 1-2 days after your injections.
I would anticipate antipyretics (acetaminophen, ibuprofen, naproxen) may help lower these risks (thoughts?).
I would anticipate antipyretics (acetaminophen, ibuprofen, naproxen) may help lower these risks (thoughts?).
Although a lot of my comments may seem negative (due to the apparent double standard likely from commercial bias to sell their product), I do anticipate once more data are known (increase denominator), we will see efficacy in all the subgroups and endpoints.
In conclusion:
Personally, I believe the benefits do outweigh the risks.
I have signed up to receive the COVID vaccine, once I am eligible. Not just for my benefit, but for my family, friends and our community.
If we all do our part, herd immunity can help eliminate COVID-19.
Personally, I believe the benefits do outweigh the risks.
I have signed up to receive the COVID vaccine, once I am eligible. Not just for my benefit, but for my family, friends and our community.
If we all do our part, herd immunity can help eliminate COVID-19.
Anticipating (hoping) other vaccines will have similar results, thanks to so many for getting us vaccines so quickly.
Pharmaceutical companies (Pfizer, Moderna, AZ)
Volunteers enrolling in the trials
Federal Gov't to financially support Warp Speed
Healthcare workers.
Pharmaceutical companies (Pfizer, Moderna, AZ)
Volunteers enrolling in the trials
Federal Gov't to financially support Warp Speed
Healthcare workers.

 Read on Twitter
Read on Twitter!["Previous SARS-CoV-2 Infxn" (briefing doc)Visit #1 (D0), ~3% positive (+ hx or asymptomatic) 526/18163 [2.9%] vs 567/18287 [3.1%])(p27)"Outcome" day (~D28): pts w/o evid of infxn prior to 7d after dose #2: ~86% (p18); therefore, this means ~14% asymptomatic positive, correct? "Previous SARS-CoV-2 Infxn" (briefing doc)Visit #1 (D0), ~3% positive (+ hx or asymptomatic) 526/18163 [2.9%] vs 567/18287 [3.1%])(p27)"Outcome" day (~D28): pts w/o evid of infxn prior to 7d after dose #2: ~86% (p18); therefore, this means ~14% asymptomatic positive, correct?](https://pbs.twimg.com/media/Eo74_Y1W4AUQ3Jo.png)
!["Previous SARS-CoV-2 Infxn" (briefing doc)Visit #1 (D0), ~3% positive (+ hx or asymptomatic) 526/18163 [2.9%] vs 567/18287 [3.1%])(p27)"Outcome" day (~D28): pts w/o evid of infxn prior to 7d after dose #2: ~86% (p18); therefore, this means ~14% asymptomatic positive, correct? "Previous SARS-CoV-2 Infxn" (briefing doc)Visit #1 (D0), ~3% positive (+ hx or asymptomatic) 526/18163 [2.9%] vs 567/18287 [3.1%])(p27)"Outcome" day (~D28): pts w/o evid of infxn prior to 7d after dose #2: ~86% (p18); therefore, this means ~14% asymptomatic positive, correct?](https://pbs.twimg.com/media/Eo75ca-XMAAwqBE.png)
![Primary Outcome [+ COVID (symptomatic disease)]Pts w/o the asymptomatic carriers on ~D28 (7d after 2nd dose): 8 vs 162 (vax vs placebo respectively)(p24)."Vaccine Efficacy" (VE) = 154/162 = 95% didn't get COVID in vax arm compared to placebo arm. Primary Outcome [+ COVID (symptomatic disease)]Pts w/o the asymptomatic carriers on ~D28 (7d after 2nd dose): 8 vs 162 (vax vs placebo respectively)(p24)."Vaccine Efficacy" (VE) = 154/162 = 95% didn't get COVID in vax arm compared to placebo arm.](https://pbs.twimg.com/media/Eo79dxFXEAASWfw.png)