Tens of thousands of people have already been vaccinated as part of Covid clinical trials. We haven't seen any significant safety concerns so far, and the FDA just gave an emergency approval for the Pfizer vaccine. Let's talk more about vaccine safety. 1/
An independent committee conducts their own analysis of clinical trial data for all vaccine candidates. Covid vaccines are no exception, and this week the committee released its analysis of the Pfizer vaccine. Vaccine efficacy in preventing Covid after two doses was 95%. 2/
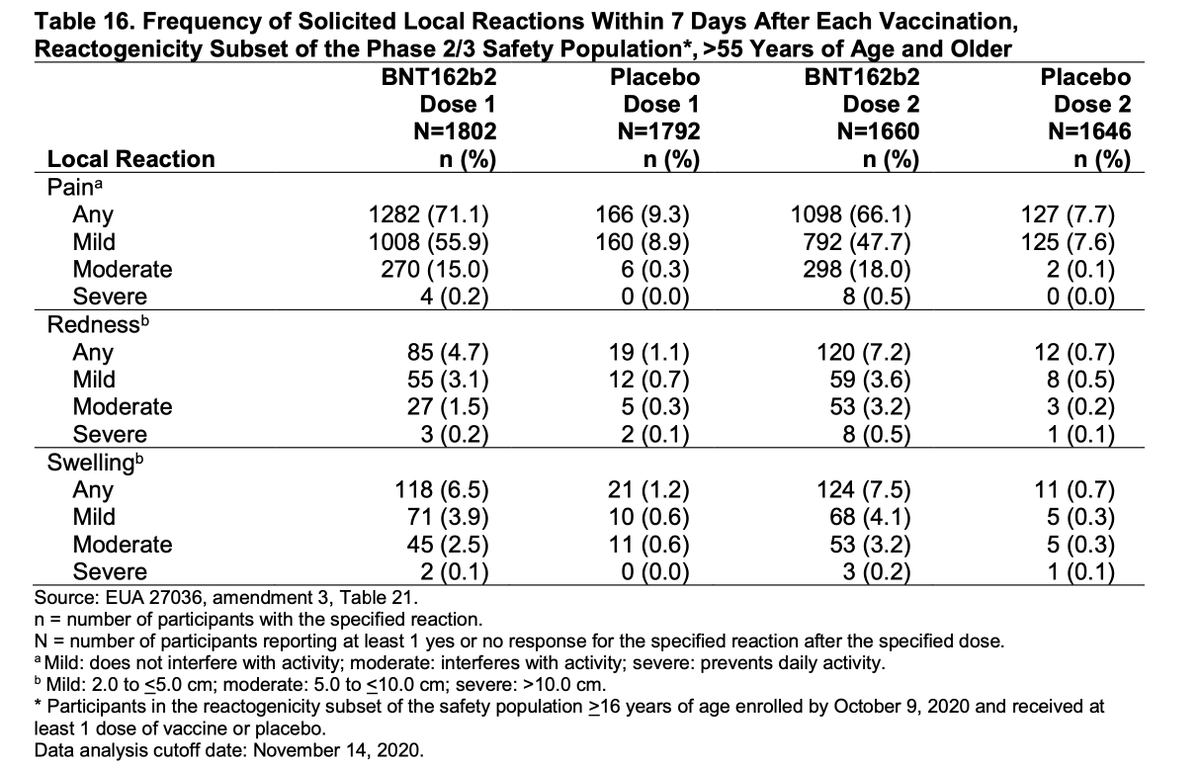
After the second shot, most people in the clinical trial had headache and fatigue and 10-15% felt feverish; this went away within a day or so, and is a sign the vaccine is working. No serious adverse reactions have been identified. 3/
Yet when millions of people are vaccinated, rare adverse effects could become apparent. We can anticipate that some people will get sick after vaccination. The challenge will be to determine whether these illnesses are purely coincidental or if they're related to the vaccine. 4/
Even if a harmful immune reaction occurs in just one in 100,000 vaccinated people, it would need to be rapidly identified, effectively treated when possible, and transparently discussed. 5/
Transparency is essential to build trust, address concerns, and counter false information. We must consistently share what we know, how we know it, what we don't know, and what we're doing to find out. 6/
Vaccines are coming, and they offer us hope for an end to the pandemic. But for most Americans, they're not coming soon enough. In the months ahead, we still have to wear masks, watch our distance, and wash our hands. 7/7

 Read on Twitter
Read on Twitter