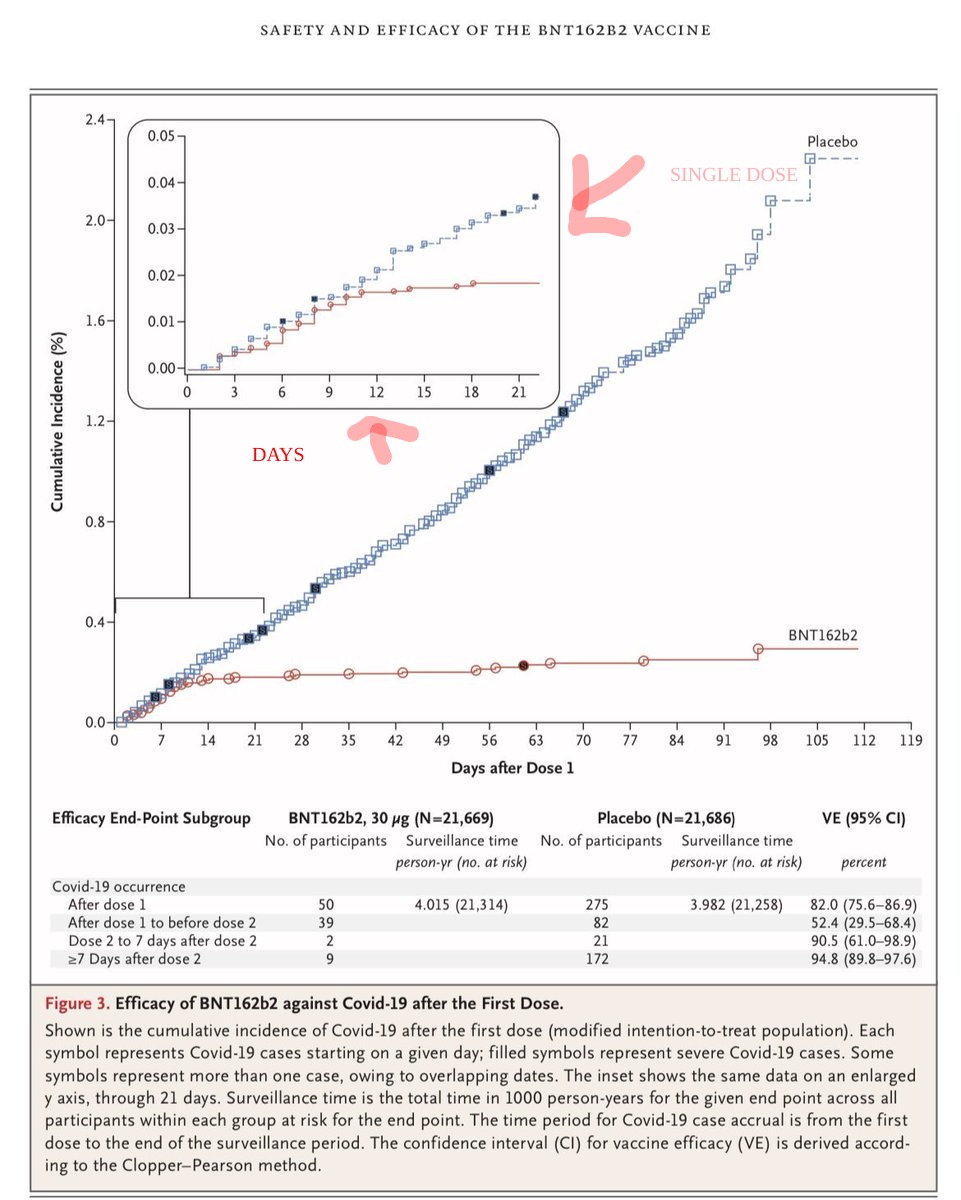
The BioNTech/Pfizer data is out—NEJM & FDA report. I understand this wasn't in the design & the long-term protection from single dose is open question. But given this sharp drop after 14 days after one dose, can someone explain why single dose isn't very very high on the agenda?
Yes, ideally there would have been a single-dose study as well. But here we are, and with a shortage that will mean many more people will die and everything else will be delayed. Personally, looking at that data, I'd quickly take one dose to give someone else a chance at one.
Maybe. There are 1256 people who did not receive the second dose in the trial. Yes, small group but what happened to them? I get it, ideally, we have a single dose study. Not so ideally, we have this data and a severe shortage. Seems worth a discussion. https://twitter.com/Valentine721/status/1337048321026875394
Pfizer reports "52.4% efficacy" for single dose but that includes the first seven days, before things kick in, when most new infections happened. At 10-12 days, the chart (eyeballing, no underlying data yet) looks ~80-85% efficacy. Unpleasant trade-off but reality is unpleasant.
I'm hoping for immunology/virology people to tell us the potential trade-offs here. We know one side: due to shortages, hundreds of millions of people will not get vaccinated anytime soon. What does the calculation on the other side look like?
At a minimum minimum, there should be an immediate single-dose trial launched, like yesterday. We sadly have a raging epidemic and will get results quickly but I think not giving this real thought now—and an explanation to the public—would be a grave mistake, given the stakes.
Yeah, but given the stakes, "we don't know for sure so we won't vaccinate hundreds of millions" is not an acceptable answer. Shortage is a terrible trade-off, too. Why didn't they/we immediately launch a single dose trial after prelim data? They knew. https://twitter.com/sdbaral/status/1337053741015502850
Look, folks, like all things pandemic, this is a trade-off. The immunology people should weigh in and explain to us about one side of the trade-off. The other side is a societal/ethical decision because we're bouncing potentially less efficacious against *not at all vaccinated*.
This is immediately consequential. US plans to HOLD BACK for many millions now vaccination to preserve for second dose later. @ScottGottliebMD—Pfizer board member—disagrees: "We should get as many shots in our arms as possible right now." https://www.usatoday.com/story/news/health/2020/12/07/covid-vaccine-pfizer-board-member-disagrees-us-distribution-plan/3860363001/
Let me ask this. We know older people need the booster more (from published data). But why we wouldn't launch a single-dose effort (randomized/blinded within) for <65 which can answer the questions about durability while immunizing millions more? https://twitter.com/michaelmina_lab/status/1337078184597217281
I wrote up the case for launching immediate trials to test a single-dose regime. Calling for volunteers among < 65 health-care workers for single dose and later booster seems like the minimal prudent response to this chart. We could know fairly quickly. https://zeynep.substack.com/p/vaccines-and-decision-making-with

 Read on Twitter
Read on Twitter