Like many of my industry colleagues, I'll be tweeting out some observations from today's big vaccine advisory committee meeting.
Not everything I see, but "Hmm, that was interesting..."-type stuff.
I'll try to thread my tweets to make them easier to read.
Not everything I see, but "Hmm, that was interesting..."-type stuff.
I'll try to thread my tweets to make them easier to read.
First, let's remember what's at stake: Authorization (not approval!).
An authorization decision isn't a binary (authorize/not authorize).
What we're likely talking about is authorization *and* the extent of any warnings, controls, labeling, contraindications, and monitoring.
An authorization decision isn't a binary (authorize/not authorize).
What we're likely talking about is authorization *and* the extent of any warnings, controls, labeling, contraindications, and monitoring.
FDA's pre-meeting briefing noted a few side effects of *potential* association which may warrant further monitoring. There were also lots of populations and sub-populations that weren't extensively studied (pregnant women, children, as well as persons with certain conditions).
Expect to see lots of discussion about the need to obtain further evidence, even if the data so far are really, really good. Rare side effects in a 44,000-person trial (a HUGE trial) can become more apparent once a vaccine is used in hundreds of million of people.
Actually, here's FDA's Doran Fink (Deputy Director, Division of vaccines and related products applications) making that point explicitly now:
Committee member Jeanette Lee asks a great question:
If FDA *approves* a vaccine, would that preclude the issuance of EUAs for other vaccines?
FDA's Fink: Yes, BUT there are lots of exceptions. If approved vaccine is in low supply, or new vaccine treats special sub-pops, EUA OK
If FDA *approves* a vaccine, would that preclude the issuance of EUAs for other vaccines?
FDA's Fink: Yes, BUT there are lots of exceptions. If approved vaccine is in low supply, or new vaccine treats special sub-pops, EUA OK
Another question: Can Pfizer market this vaccine under an EUA?
FDA's Fink: No, EUA statute explicitly prevents.
So, are we just going to know this vaccine as BNT162b2? Because that seems... suboptimal. Especially for patients remembering which vaccine they received.
FDA's Fink: No, EUA statute explicitly prevents.
So, are we just going to know this vaccine as BNT162b2? Because that seems... suboptimal. Especially for patients remembering which vaccine they received.
Now that I think about the above tweet a bit more, lots of EUA-granted diagnostics *have* brand names, so it's possible (probable?) that this vaccine will as well.
One to watch.
One to watch.
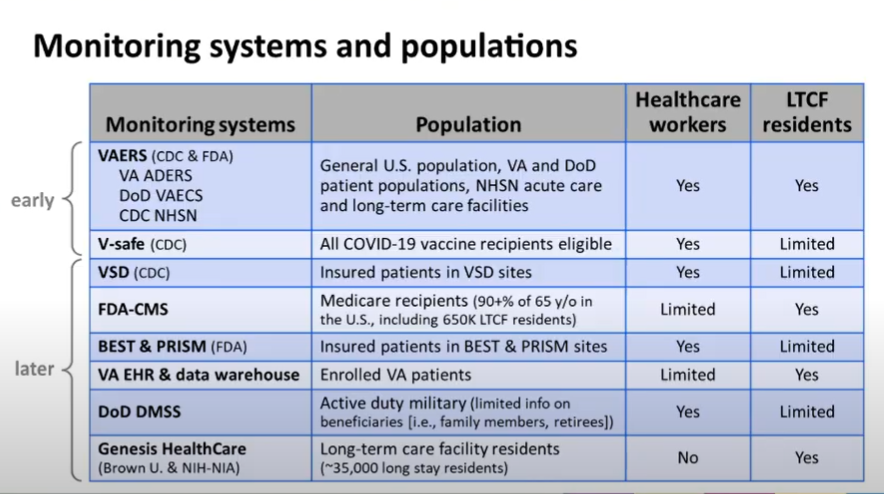
CDC's Nancy Messonnier now providing a preview of some the huge number of surveillance systems used to monitor vaccine effects after authorization:
V-Safe is (I believe) a new one based on text messaging with vaccinated essential workers.
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-09/COVID-03-Shimabukuro.pdf
V-Safe is (I believe) a new one based on text messaging with vaccinated essential workers.
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-09/COVID-03-Shimabukuro.pdf
As a side note: It drives me nuts that half of these meetings are taken up by presentations from CDC.
There is a TON of FDA-specific information that needs to be discussed, and some of these CDC presentations are tangential at best to regulatory issues (like order volume).
There is a TON of FDA-specific information that needs to be discussed, and some of these CDC presentations are tangential at best to regulatory issues (like order volume).
Steven Goodman, providing an overview of ethics of unblinding trial participants, probably giving Pfizer and FDA staff ulcers right now.
Paraphrasing: 'People with means to unblind themselves could by testing presence of spike protein using diagnostics.'
Paraphrasing: 'People with means to unblind themselves could by testing presence of spike protein using diagnostics.'
Public Citizen's Sidney Wolf says at the outset of his remarks that he supports FDA granting an EUA for the Pfizer vaccine.
Notable since Public Citizen is probably best known for being the voice against approval at most AdComms and in many Citizen Petitions.
Notable since Public Citizen is probably best known for being the voice against approval at most AdComms and in many Citizen Petitions.
Let's be honest here: If the FDA is anticipating granting an EUA within 1-7 days after today's meeting...
... nothing said during this public session is likely to change anything.
... nothing said during this public session is likely to change anything.
It's worth noting that FDA has frequently indicated that some of the public comments (and AdComm comments!) aren't especially helpful.
FDA's review process often gives deep consideration to lots of issues that are later raised superficially at the meeting.
FDA's review process often gives deep consideration to lots of issues that are later raised superficially at the meeting.
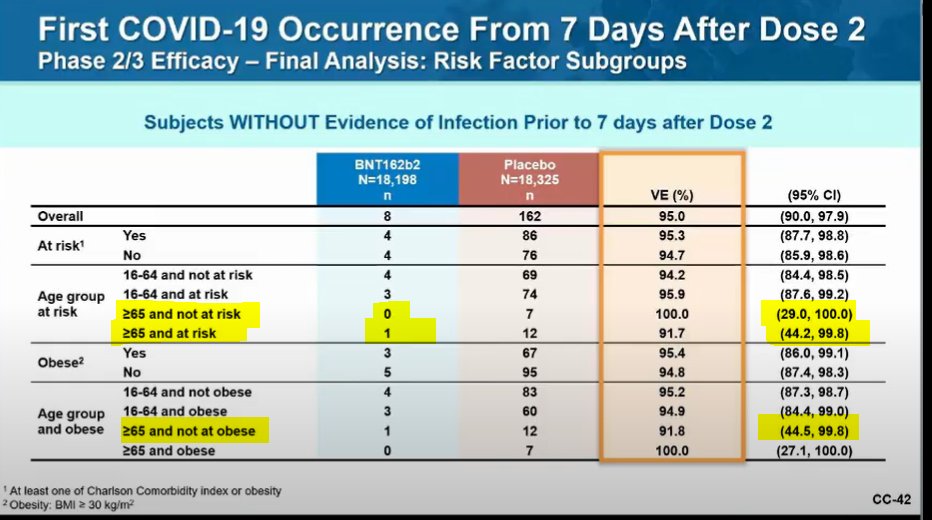
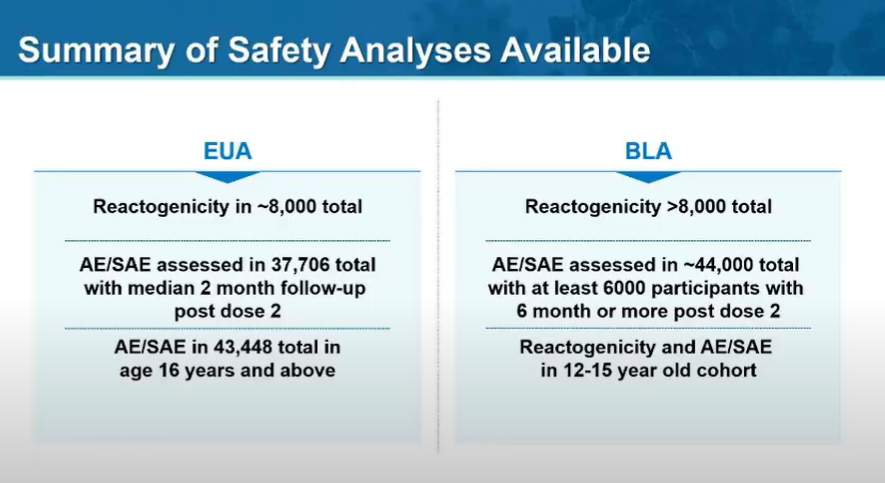
Something to keep an eye on is subgroup efficacy of the vaccine.
The trial was huge (44k+ enrollees), but individual groups and incidence of COVID was sometimes small. That's resulted in estimates w/ HUGE range in confidence interval.
One slide from Pfizer w/ a few highlights:
The trial was huge (44k+ enrollees), but individual groups and incidence of COVID was sometimes small. That's resulted in estimates w/ HUGE range in confidence interval.
One slide from Pfizer w/ a few highlights:
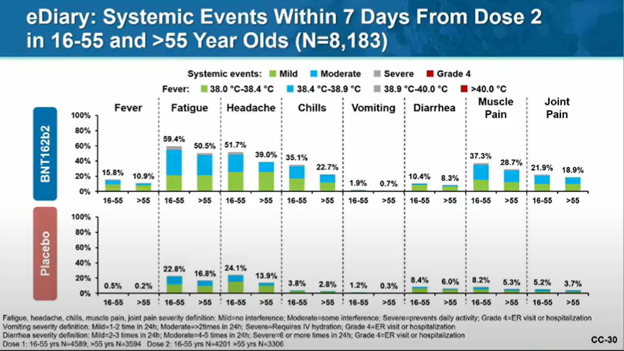
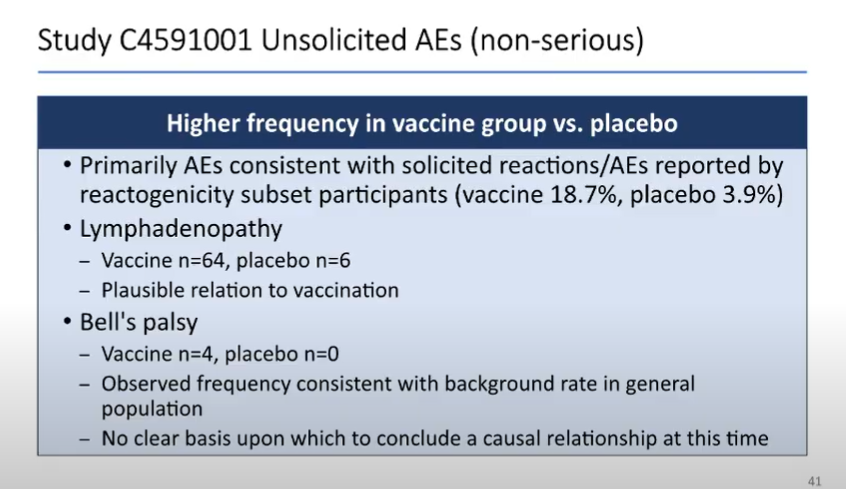
And as noted by me (and many others) previously, the general side effects of the COVID-19 vaccine are a bit higher than you might expect from a flu vaccine, but are consistent with some other vaccines.
Generally minor (and better than COVID!)
Some visual data from Pfizer:
Generally minor (and better than COVID!)
Some visual data from Pfizer:
Pfizer now providing some insight into what it's Biologics License Application (i.e., application for approval) will look like.
Says it plans to file April 2021.
Says it plans to file April 2021.
Assuming that timeline holds, that's a line in the sand for future vaccine developers. Once FDA approves a BLA, it becomes harder (though as noted earlier, not impossible) to obtain EUAs.
I would expect that BLA to be reviewed pretty quickly, almost more akin to a supplement.
I would expect that BLA to be reviewed pretty quickly, almost more akin to a supplement.
Paul Offit raises one of THE key questions:
Only about 162 cases of COVID in the trial, mostly in the placebo group. Is there any evidence of a "volunteer bias" - the idea that a trial participant might be more likely to use a mask, distance or avoid illness?
Only about 162 cases of COVID in the trial, mostly in the placebo group. Is there any evidence of a "volunteer bias" - the idea that a trial participant might be more likely to use a mask, distance or avoid illness?
Pfizer's response is that the disease incidence rate (0.7 by Offit's estimation) was roughly similar to the incidence seen in the general population.
This is easily one of the most important questions for FDA to monitor after authorization.
This is easily one of the most important questions for FDA to monitor after authorization.
Because if incidence in the trial was artificially low, that could mean once the vaccine is used in real-world populations, the rate of disease (and protection) could be different.
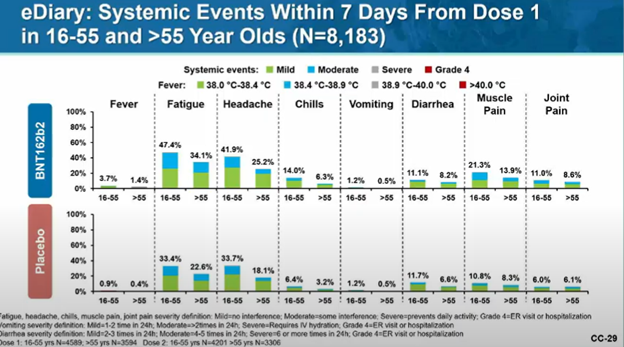
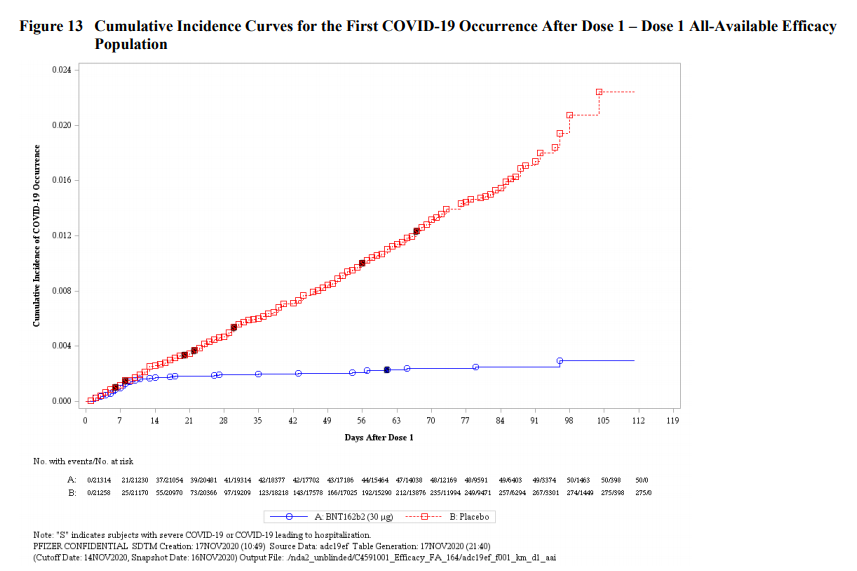
VRBPAC member Juan Gea-Banacloche asks: Based on Pfizer data, could a single dose of vaccine work compared to two dose?
Pfizer: Can't conclude that a single dose will lead to similar levels of protection. Trial wasn't designed to predict since it was designed for 2 doses.
Pfizer: Can't conclude that a single dose will lead to similar levels of protection. Trial wasn't designed to predict since it was designed for 2 doses.
That question of course refers to this graphic, which had epidemiologists everywhere this week remembering what it is like to feel joy:
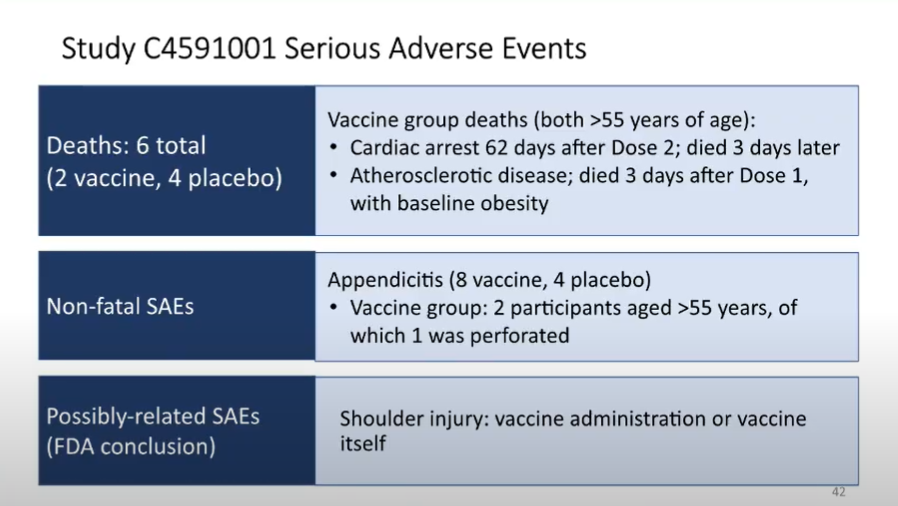
FDA's Wollensheim now talking about serious adverse events seen during the trial.
Important to note these are *associations*, and in some cases were not identified at rates higher than is expected in the general population. Still, FDA will be monitoring for further signs.
Important to note these are *associations*, and in some cases were not identified at rates higher than is expected in the general population. Still, FDA will be monitoring for further signs.
As folks have noted, if the vaccine is authorized and widely administered, people are going to be associating *everything* that happens with the vaccine.
Headache? Vaccine. Gallstones? Vaccine. etc etc
A tough job for FDA to determine causation, if it exists.
Headache? Vaccine. Gallstones? Vaccine. etc etc
A tough job for FDA to determine causation, if it exists.
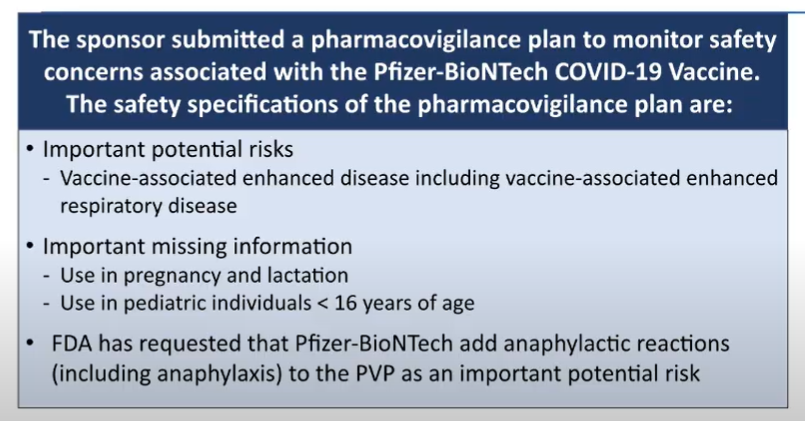
Woah, FDA breaking some news in that last slide. Says FDA asked Pfizer to add anaphylactic reactions to the pharmacovigilance plan "as an important potential risk."
The UK had flagged those risks yesterday.
The UK had flagged those risks yesterday.
For reference, FDA's Peter Marks had actually admonished UK regulators about their reaction to this very risk yesterday.
@steveusdin1's piece on this: https://www.biocentury.com/article/632581/fda-s-marks-admonishes-u-k-regulators-for-discussing-covid-vaccine-adverse-events-as-mhra-updates-guidance
@steveusdin1's piece on this: https://www.biocentury.com/article/632581/fda-s-marks-admonishes-u-k-regulators-for-discussing-covid-vaccine-adverse-events-as-mhra-updates-guidance
Which would seem to indicate that FDA isn't concerned about them enough to put them on the product label (but maybe that changes w/ UK and Canadian data this week), but wants to keep a close eye on it long-term.
FDA's Doran Find says Pfizer, regulators are still looking for more conclusive data about effects of vaccine in persons who are pregnant (knowingly or unknowingly).
Says they don't have data yet. Will soon have DART studies (reproductive toxicity), but no concerns right now.
Says they don't have data yet. Will soon have DART studies (reproductive toxicity), but no concerns right now.
FDA's Gruber now confirming that in addition to Pfizer postmarketing study, FDA and Pfizer's vaccine label will include warning about potential for anaphylaxis due to allergy to ingredients in vaccine. Sites will need to have drugs on hand to reverse an anaphylactic event. (1/2)
Cody Meissner notes that this is actually a common requirement for most vaccines. FDA's Gruber says she is still not sure that the vaccine and allergic reactions "are related."
Says FDA has been in contact with the UK's MHRA for more detail. (2/2)
Says FDA has been in contact with the UK's MHRA for more detail. (2/2)
Now the committee is touching on another key issue: Even if the vaccine prevents COVID-19, does it prevent transmission (or "carriage") of the virus to others?
Pfizer says it plans to study more using PCR tests. Preliminary data from primate studies promising.
Pfizer says it plans to study more using PCR tests. Preliminary data from primate studies promising.
Until this question is answered, it's probably worth maintaining the habit of remaining masked even after vaccination.
For the same reason you should wear masks now: To protect others from the possible spread of COVID.
For the same reason you should wear masks now: To protect others from the possible spread of COVID.
Another aside: This AdComm spent like 5 hours this morning basically reading from slides that were already made public, and now the Acting Chair, Arnold Monto, is limiting the challenging questions from the experts, saying they're short on time.
Utterly asinine.
Utterly asinine.
VRBPAC member Ofer Levy now digging into pediatric vaccination. The vaccine trial didn't include anyone under 17.
Pfizer said it's going to extrapolate some data, starting testing in older children, and then start further testing based on "judicious" dosing and testing.
Pfizer said it's going to extrapolate some data, starting testing in older children, and then start further testing based on "judicious" dosing and testing.
And we have now reached the headline-making part of the meeting, where they actually vote on this question:
Committee members now teeing up the vote.
Cody Meissner recounting that when he looked at adverse events associated with vaccines, basically all of them happened within a time period of 6 weeks. One exception was a life polio vaccine, and the mRNA is not a live vaccine.
Cody Meissner recounting that when he looked at adverse events associated with vaccines, basically all of them happened within a time period of 6 weeks. One exception was a life polio vaccine, and the mRNA is not a live vaccine.
There's an interesting argument here about which patients should be vaccinated, and specifically younger patients.
The argument: Without data on 16-17 year-olds, it's hard to tell which side effects *weren't* caused by a vaccine. (1/2)
The argument: Without data on 16-17 year-olds, it's hard to tell which side effects *weren't* caused by a vaccine. (1/2)
Philip Krause of the FDA points to incidents where harm is mistakenly attributed to a vaccine, when in fact it played no role.
Clinical trial data helps regulators to address this. (2/2)
Clinical trial data helps regulators to address this. (2/2)
VRBPAC member Mark Sawyer raises an interesting point:
Why not delay the recommendation for 16-17 year-olds for a few months since they are likely to be among the last to be vaccinated anyways? Continue to collect data in the meantime.
Why not delay the recommendation for 16-17 year-olds for a few months since they are likely to be among the last to be vaccinated anyways? Continue to collect data in the meantime.
I should note that this is a *very* contentious point. Others, including Offit, say they don't need to know "Everything - just enough."
Offit says efficacy, safety seem adequate, and there are plenty of sick/ill/unhealthy kids with real health risks who could benefit.
Offit says efficacy, safety seem adequate, and there are plenty of sick/ill/unhealthy kids with real health risks who could benefit.
As an aside: On this meeting, there are collectively 31 PhDs and 17 Ph.Ds...
....some of the smartest minds on vaccine science in the world...
... and almost none of them know how to unmute themselves when they start talking.
....some of the smartest minds on vaccine science in the world...
... and almost none of them know how to unmute themselves when they start talking.
Ok, we're starting voting now.
There's some contention about the wording of the question. It's clear some people want to vote for the vaccine, but aren't comfortable recommending it for 16-17 year-olds.
There's some contention about the wording of the question. It's clear some people want to vote for the vaccine, but aren't comfortable recommending it for 16-17 year-olds.
And we have a vote! (On a SPECIFIC question - not the vaccine a a whole)
17 in favor.
4 against.
1 Abstain
17 in favor.
4 against.
1 Abstain
Persons voting No:
Kurilla, Chartterjee, Fuller, Kim
Abstain: Meissner
Kurilla, Chartterjee, Fuller, Kim
Abstain: Meissner
And... FDA just ends the committee hearing without asking any of the "No" votes for an explanation.
It *seemed* like most of the "no" votes were about the access for 16-17 year-olds, but no one had a chance to explain.
It *seemed* like most of the "no" votes were about the access for 16-17 year-olds, but no one had a chance to explain.
Alright, I'm going to take a break to write up my full thoughts for AgencyIQ subscribers, but feel free to ask questions as you have them and I'll try to answer them here.
Overall, a very good meeting for Pfizer - one that raised few questions about the vaccine or its safety.
Overall, a very good meeting for Pfizer - one that raised few questions about the vaccine or its safety.

 Read on Twitter
Read on Twitter