Take a listen live to the FDA's independent committee (VRBPAC) meeting re Pfizer vaccine. https://twitter.com/CNBC/status/1337034952383328259
In case you haven't seen it- here is the FDA brief with the details on all the Pfizer vaccine studies to date along with study methodology and results. https://www.fda.gov/media/144245/download
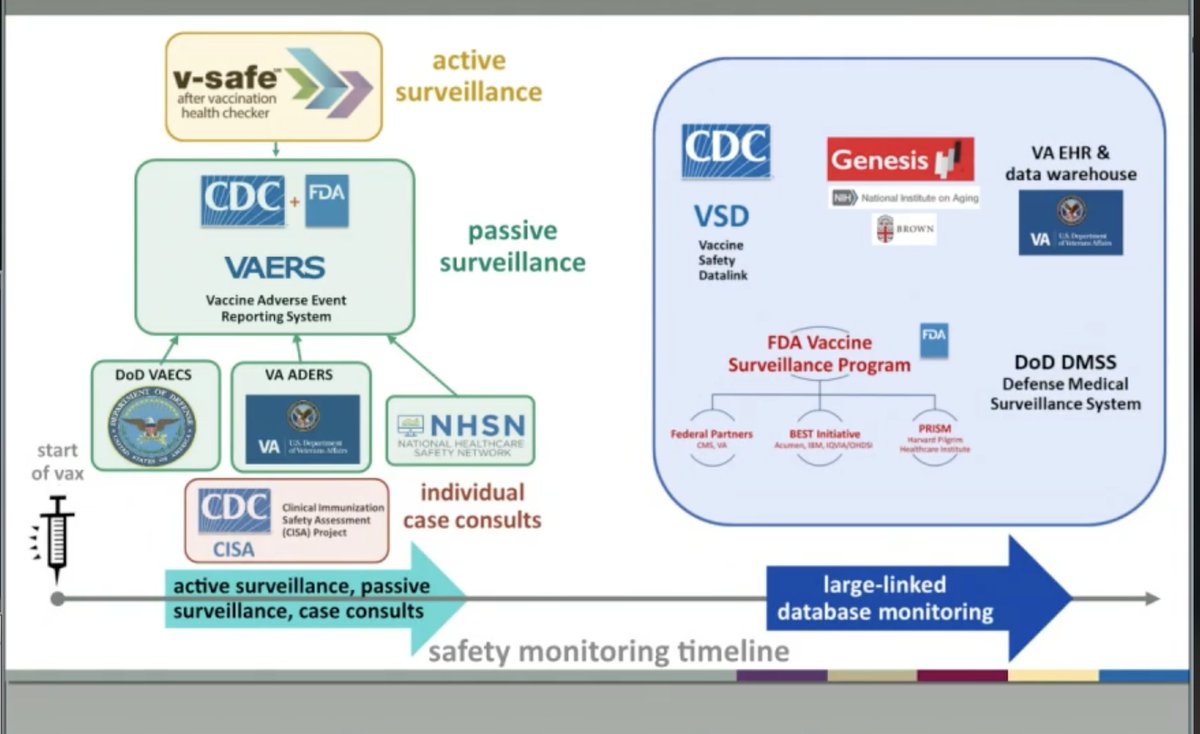
Now @DrNancyM_CDC presents post EUA safety monitoring plan, incl V-SAFE, new active surveillance system that's text based where vaccinated can submit possible adverse effects & CDC reaches out to them for details. AND new programs enhancing VAERS monitoring of HCWs and LTFs.
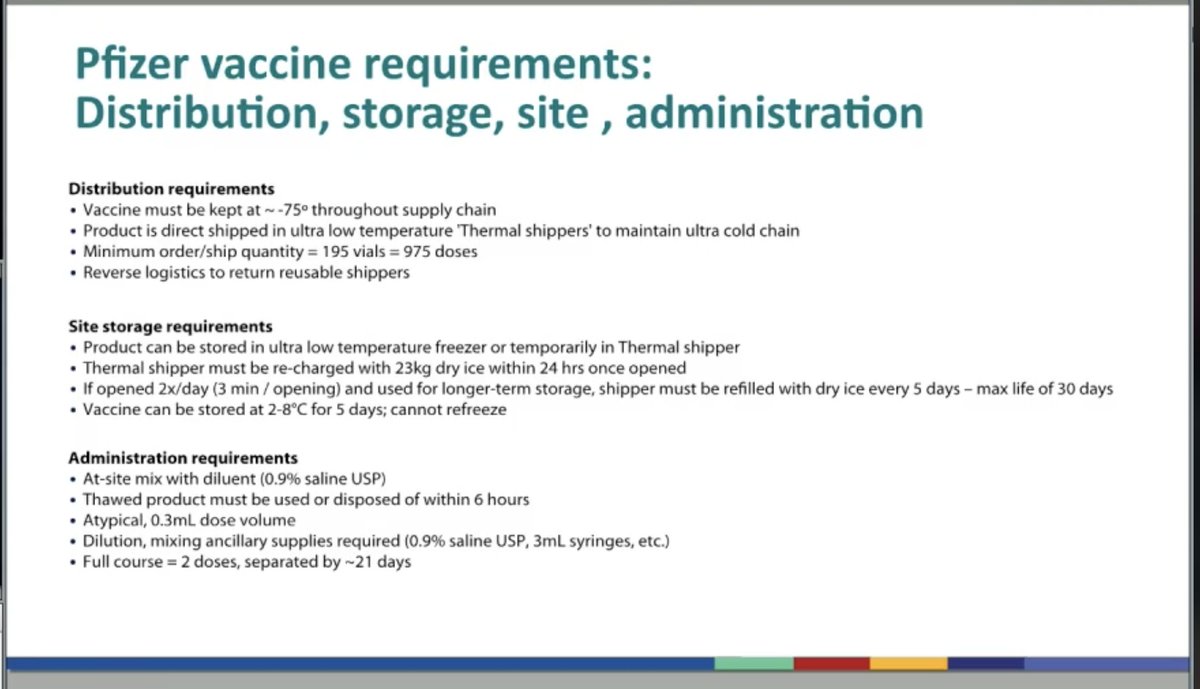
Now Dr Anita Patel @CDCgov presents distribution plans
Great question from Dr. Ofer Levy about whether US vaccine safety surveillance will coordinate with global systems.
@DrNancyM_CDC says there is WHO coordinated group that will look & share safety & efficacy across the globe.
@DrNancyM_CDC says there is WHO coordinated group that will look & share safety & efficacy across the globe.
Dr Steven Godoman says future placebo controlled vaccine trials may not be unethical but they may not be feasible.
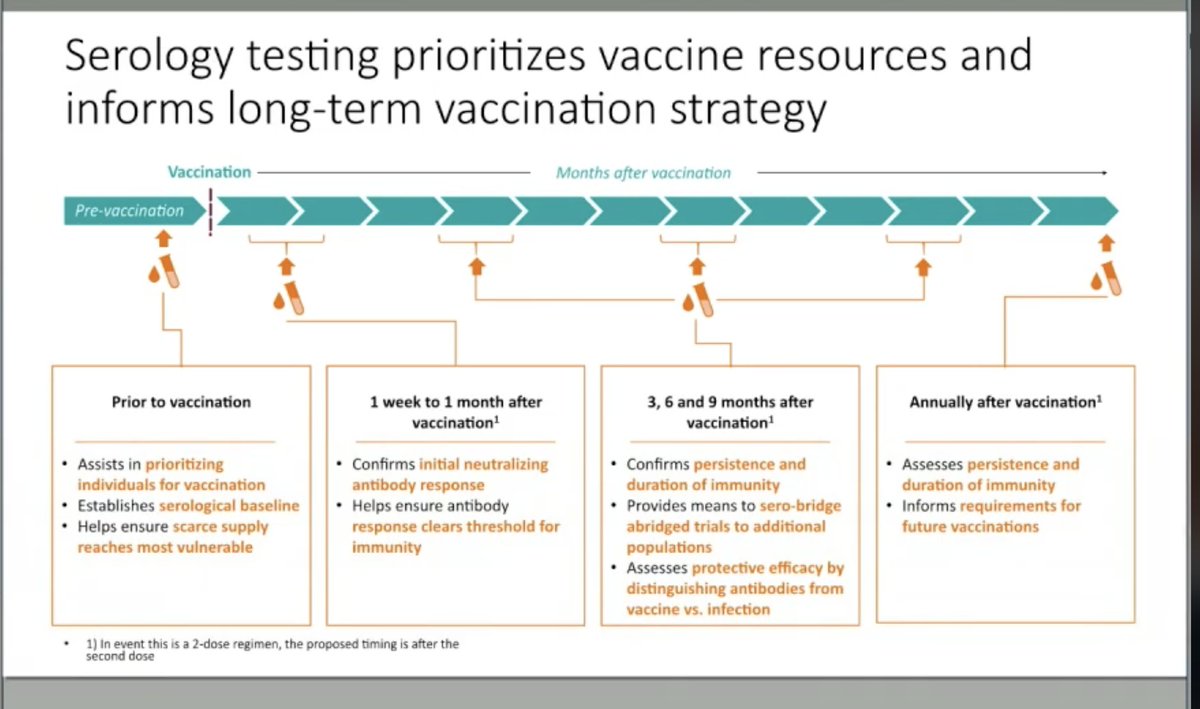
Awesome presentation by @angie_rasmussen on use of serology to help inform vaccine strategy
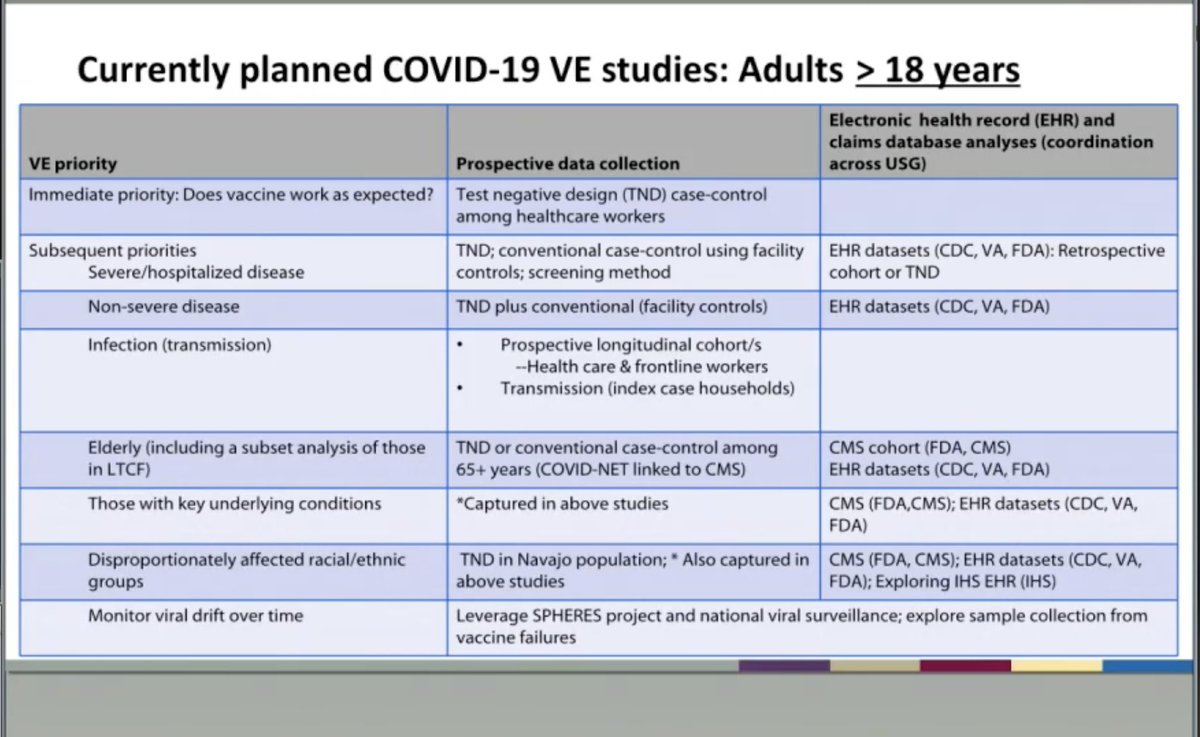
Important conversation re 8 vaccine failures (those who got vaccine but still got disease)- @pfizer was asked about whether they had sequenced the viruses and they said they plan to do this analysis but do not currently have the data.
Both patient & viral characteristics important to answer vaccine failure question.
I hope they also look at all possible logistic failures as well since they become magnified in real life.
I hope they also look at all possible logistic failures as well since they become magnified in real life.

 Read on Twitter
Read on Twitter