HUGE—Oxford Jenner Institute Director Adrian Hill, who oversees Oxford-AstraZeneca #COVID19 vaccine research, warns that if FDA doesn’t approve Oxford vaccine now but waits for the end of Oxford’s 2nd Phase 3 trial, vaccine won’t be available in US until “the middle of [2021]."
2) To be clear, Oxford has reported its Phase 3 trial. And it’s even the first to fully peer review publish Phase results. https://twitter.com/drericding/status/1336720851165728770
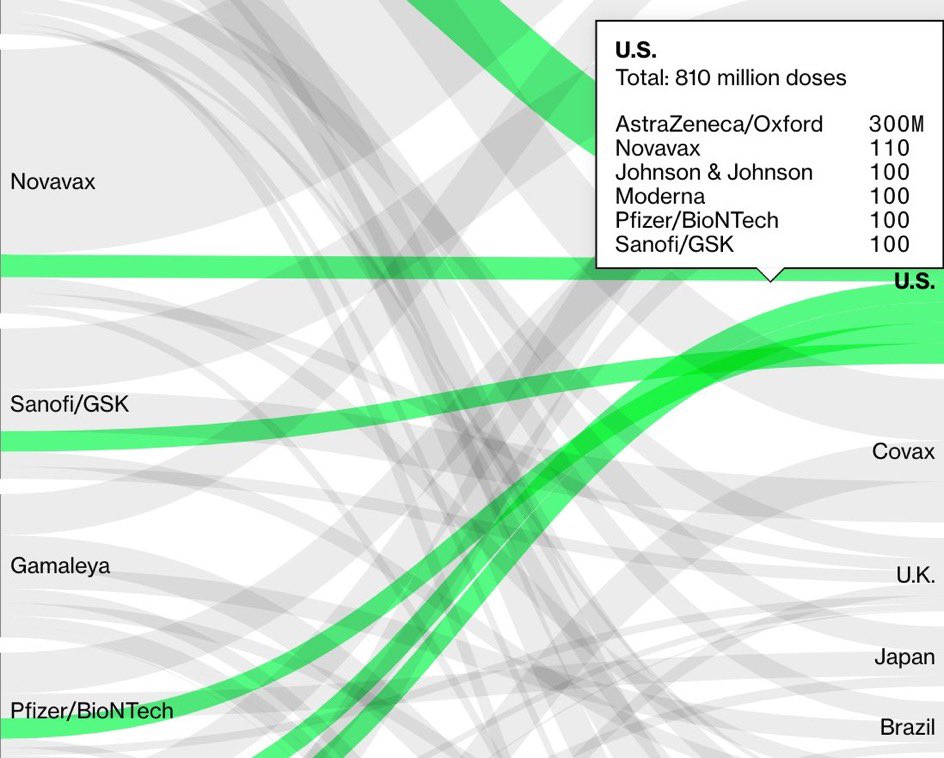
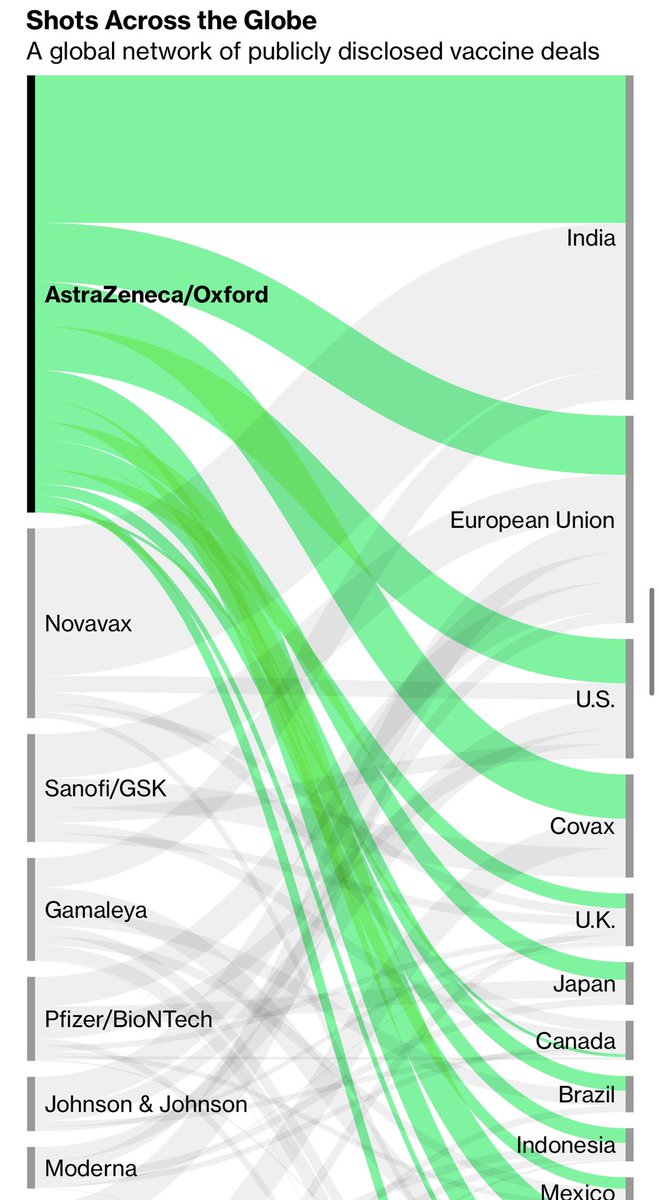
3) the thing is that US’s vaccine order is hugely dependent on Oxford AstraZeneca vaccine. 300 million doses of it, for 150 million people. And we can’t get more Pfizer/BioNTech until June earliest since Trump WH has waived ordering more.
4) And the Oxford / AstraZeneca vaccine is **the** top vaccine on order worldwide. Hence this affects the whole world. And many countries follow FDA’s lead.
5) That said, Oxford- AstraZeneca ran into a bit of problem with some of the dosing for part of the trial due to a contractor error. Part of the trial got an initial half dose. But incidentally that half dose was what found the 90% efficacy. Hence some demanding Oxford repeat it. https://twitter.com/drericding/status/1331913357033664512
6) To be clear, it’s **NOT** a safety issue that is making Oxford repeat the low dose trial. It’s because critics say they stumbled on the low dose 90% efficacy result by dumb luck. It wasn’t a safety concern per se. it was a “prove it’s really 90%” challenge.
7) Half-dose of Oxford vaccine was NOT a 'mistake' and was known about before people got the jabs, scientists say as project chief says UK regulator could approve the 90% effective combination despite small study. https://www.google.com/amp/s/www.dailymail.co.uk/news/article-9033881/amp/Half-dose-Oxford-vaccine-NOT-mistake-known-people-got-jabs-scientists-say.html

 Read on Twitter
Read on Twitter![HUGE—Oxford Jenner Institute Director Adrian Hill, who oversees Oxford-AstraZeneca #COVID19 vaccine research, warns that if FDA doesn’t approve Oxford vaccine now but waits for the end of Oxford’s 2nd Phase 3 trial, vaccine won’t be available in US until “the middle of [2021]." HUGE—Oxford Jenner Institute Director Adrian Hill, who oversees Oxford-AstraZeneca #COVID19 vaccine research, warns that if FDA doesn’t approve Oxford vaccine now but waits for the end of Oxford’s 2nd Phase 3 trial, vaccine won’t be available in US until “the middle of [2021]."](https://pbs.twimg.com/media/Eo1WM_aXcAIeuVV.jpg)