Today @US_FDA released @pfizer's #COVID19Vaccine phase 3 trial data.
Link to the Pfizer-BioNTech document (92 pages): https://www.fda.gov/media/144246/download
Link to the FDA briefing document (53 pages): https://www.fda.gov/media/144245/download
I am optimistic about these results...
Thread...
Link to the Pfizer-BioNTech document (92 pages): https://www.fda.gov/media/144246/download
Link to the FDA briefing document (53 pages): https://www.fda.gov/media/144245/download
I am optimistic about these results...
Thread...

VACCINE SEQUENCE
The Pfizer-BioNTech COVID-19 Vaccine, BNT162b2 (30 μg), is administered intramuscularly (IM) as a series of two 30 μg doses (0.3 mL each) 21 days apart.
The Pfizer-BioNTech COVID-19 Vaccine, BNT162b2 (30 μg), is administered intramuscularly (IM) as a series of two 30 μg doses (0.3 mL each) 21 days apart.
STUDY DESIGN:
Randomized, double-blind placebo-controlled trial
SUBJECT DISPOSITION
18,904 participants received vaccine (94.2% completed both doses)
18,892 participants received placebo (94.1% completed both doses)
Randomized, double-blind placebo-controlled trial
SUBJECT DISPOSITION
18,904 participants received vaccine (94.2% completed both doses)
18,892 participants received placebo (94.1% completed both doses)
DEMOGRPAHICS
49% Females
82% White
10% African American
4% Asian
<3% other racial groups
26% Hispanic/Latino
Geographically, 77% , 15%
, 15%  , 6%
, 6%  , 2%
, 2% 
Age: Everyone >16 years (children <16 were not included), median age was 52 years (22% >65 years)
49% Females
82% White
10% African American
4% Asian
<3% other racial groups
26% Hispanic/Latino
Geographically, 77%
 , 15%
, 15%  , 6%
, 6%  , 2%
, 2% 
Age: Everyone >16 years (children <16 were not included), median age was 52 years (22% >65 years)
DEMOGRAPHICS CONT'D
Most frequently reported co-morbidities:
35% obesity
25% hypertension
8% diabetes
8% chronic pulmonary disease
Limited numbers of immunocompromised patients.
Most frequently reported co-morbidities:
35% obesity
25% hypertension
8% diabetes
8% chronic pulmonary disease
Limited numbers of immunocompromised patients.
EFFICACY (i.e. EFFECTIVENESS):
1st dose 52% efficacy
52% efficacy
...(21 days interim)...
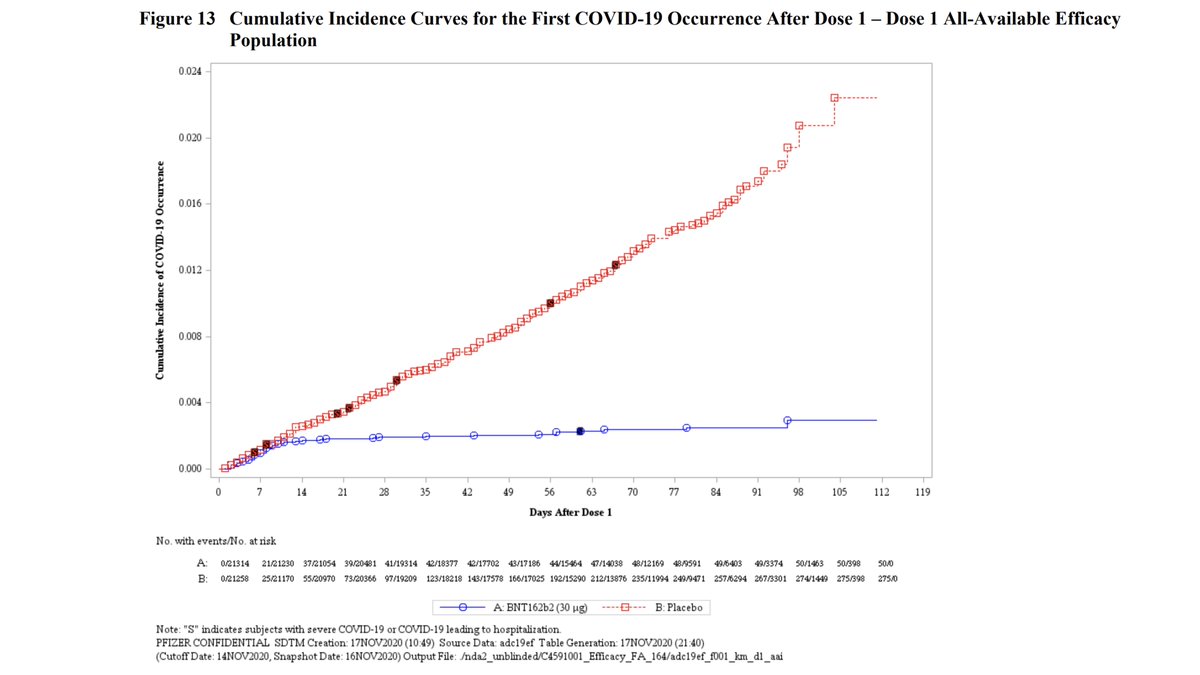
2nd dose 95% efficacy (Wow!!!)
95% efficacy (Wow!!!)
No meaningful differences btwn people of different ages or ethnicities
1st dose
 52% efficacy
52% efficacy ...(21 days interim)...
2nd dose
 95% efficacy (Wow!!!)
95% efficacy (Wow!!!)No meaningful differences btwn people of different ages or ethnicities
SAFETY:
Safety data is based on ~ 38,000 participants.
Most adverse events were common but mild:
Injection site rxns (84%)
Fatigue (63%)
Headache (55%)
Muscle pain (38%)
Chills (32%)
Joint pain (24%)
Fever (14%)
Chance of any serious adverse event was less than 0.5%
Safety data is based on ~ 38,000 participants.
Most adverse events were common but mild:
Injection site rxns (84%)
Fatigue (63%)
Headache (55%)
Muscle pain (38%)
Chills (32%)
Joint pain (24%)
Fever (14%)
Chance of any serious adverse event was less than 0.5%
SAFETY (CONT'D):
Serious adverse events, while uncommon (<0.5%), represented medical events that occur in the general population at similar frequency as observed in the study. No specific safety concerns were identified in subgroup analyses by age, race, ethnicity, comorbidities
Serious adverse events, while uncommon (<0.5%), represented medical events that occur in the general population at similar frequency as observed in the study. No specific safety concerns were identified in subgroup analyses by age, race, ethnicity, comorbidities
SAFETY (CONT'D):
4 people in vaccine group (out of 18,904) developed Bell's palsy, compared to 0 in placebo group. Not very concerning b/c this frequency is similar to background rate in general population. FDA will recommend surveillance for Bell’s palsy w/ vaccine deployment.
4 people in vaccine group (out of 18,904) developed Bell's palsy, compared to 0 in placebo group. Not very concerning b/c this frequency is similar to background rate in general population. FDA will recommend surveillance for Bell’s palsy w/ vaccine deployment.
SAFETY (CONT'D)
6 deaths occurred during the trial: 4 in placebo group, 2 in vaccine group. These deaths were NOT related to the vaccine.
6 deaths occurred during the trial: 4 in placebo group, 2 in vaccine group. These deaths were NOT related to the vaccine.
UNKNOWNS:
? Duration of protection
? Efficacy against transmission (wear your masks!)
? Efficacy & safety among several subpopulations including kids <16 years old, pregnant/lactating women, immunocompromised (too few enrolled to make meaningful conclusions)
? Duration of protection
? Efficacy against transmission (wear your masks!)
? Efficacy & safety among several subpopulations including kids <16 years old, pregnant/lactating women, immunocompromised (too few enrolled to make meaningful conclusions)
Since there is insufficient data to determine safety of the vaccine in immunocompromised individuals, I suspect the FDA may not authorize it in these patients. This is why it is critical for the rest of us to be immunized, to increase herd immunity and protect the vulnerable.
The @US_FDA Advisory Committee Meeting to discuss the @pfizer #COVID19Vaccine is scheduled Dec 10, 2020. The FDA will likely authorize the vaccine for emergency use.
As for me, the day I am eligible for this vaccine, I will readily stand in line with my sleeve rolled up.
As for me, the day I am eligible for this vaccine, I will readily stand in line with my sleeve rolled up.

 Read on Twitter
Read on Twitter